Reporte de caso
← vista completaPublicado el 8 de mayo de 2023 | http://doi.org/10.5867/medwave.2023.04.2664
Trasplante exitoso de células madre después de nelarabina, asparaginasa pegilada, vincristina, doxorrubicina y prednisona en leucemia linfoblástica aguda precursora de células T refractaria temprana: reporte de caso
Successful stem cell transplantation after nelarabine, pegylated asparaginase, vincristine, doxorubicin, and prednisone in refractory early T-cell precursor acute lymphoblastic leukemia: A case report
Abstract
Early T-cell precursor Acute Lymphoblastic Leukemia (ALL) has a dismal prognosis. Nelarabine is a purine nucleoside analog that increases the apoptosis rate in T-cell lymphoblasts. We present a 30-year-old patient diagnosed with T-cell ALL. He was a high-risk patient because of an early precursor phenotype and a complex karyotype that had been refractory to three previous lines of treatment. He started a course of nelarabine (1500 mg/m for three days), pegylated-asparaginase, doxorubicin, vincristine, and prednisone (Nelarabine Peg-Asp AdmVP). He reached complete remission and received an allogeneic sibling hematopoietic stem cell transplant with fludarabine, total body irradiation, and cyclophosphamide as the conditioning regimen. He developed a pulmonary mycosis, which resolved, and grade-2 neurotoxicity in his upper and lower limbs. He was discharged after 40 days and to date remains with 23 months of complete remission. The Nelarabine Peg-Asp AdmVP regimen seems to be effective and safe. Further research is needed to establish it as an induction treatment in refractory early T-cell precursor acute lymphoblastic leucemia.
Main messages
- Early T-cell precursor acute lymphoblastic leukemia has a dismal prognosis.
- Nelarabine, pegylated-asparaginase, doxorubicin, vincristine, and prednisone regimen seems effective and safe, and could be used as induction treatment in refractory early T-cell precursor acute lymphoblastic leukemia.
- This work describes a successful treatment for a high-risk patient presenting refractory early T-cell precursor acute lymphoblastic leukemia.
- While more clinical trials are needed, our case shows the effectiveness of the presented treatment.
Introduction
Early T-cell precursor Acute Lymphoblastic Leukemia (ALL) has a dismal prognosis compared to B-cell precursor ALL. A high percentage of patients are resistant to corticosteroids and have positive minimal residual disease at day + 29. Many of these patients also have induction treatment failure and only 20% 5-year survival [1,2,3]. Nelarabine is a pro-drug of deoxyguanosine analog 9-beta-D arabinofuranosylguanine (ara-G); it accumulates inside the T-lymphoblasts and prevents DNA replication in the G1/S phase and causes apoptosis, with selective cytotoxicity on these cells. It has minimal toxicity and is FDA-approved to treat B or T-cells ALL in the setting of relapsed or refractoriness after two previous lines of chemotherapy [4]. However, some pediatrics protocols have excluded this drug due to neurotoxicity concerns. Given these toxicity concerns and high treatment costs, nelarabine is generally used in high-risk relapsed or refractory patients. We report a patient with early T-cell precursor ALL refractory to multiple chemotherapy regimens who was successfully re-induced with nelarabine in combination with a Japanese multi-drug protocol containing L-asparaginase, vincristine, doxorubicin, and prednisone. [5]
Clinical presentation
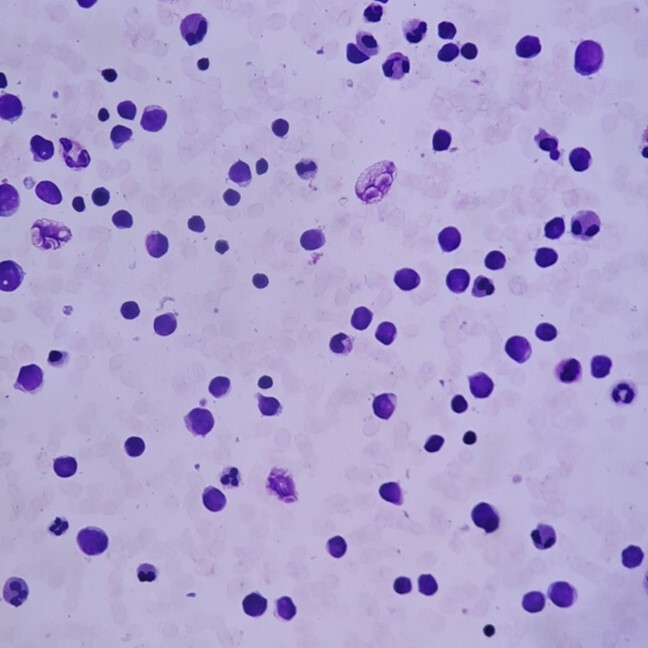
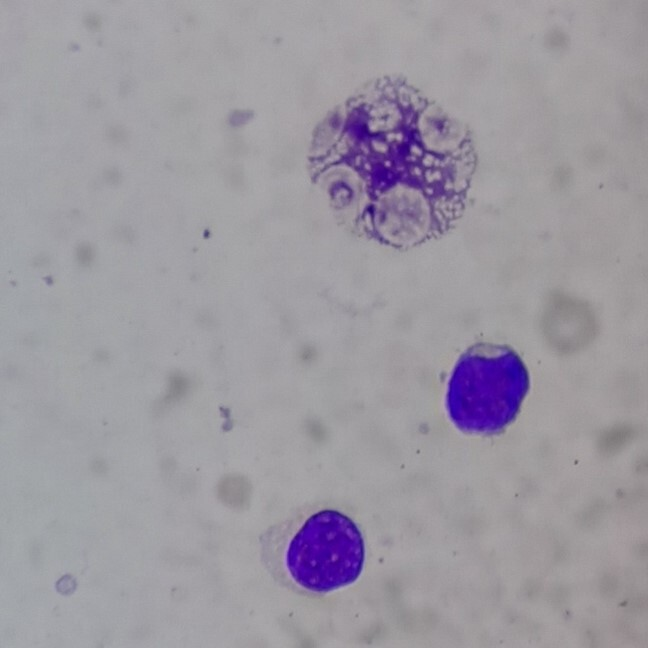
In January 2020, a 30-year-old man was diagnosed with early T-cel ALL. He was admitted for having fatigue and fever for three weeks. His complete blood count showed a hemoglobin of 13.6 g/dL, platelets 10 x 109/L, and a white blood cell count of 54 200 mm3 with 36% lymphoid blasts. Computed tomography showed bilateral axillary and hilar lymphadenopathy and splenomegaly. No mediastinal masses were detected. In Figure 1 , bone marrow aspiration shows 57.9% T-cell lymphoblasts with early T-cell precursor phenotype: CD 34 (+) and nTDT (-). T-lineage markers CD7 (+), CyCD3 (+), no B-lineage markers (CD19 (-), CyCD79a (-)) nor myeloid markers (CyMPO (-), CD13 (-)) were detected. Figure 2 shows an L1 medium-sized lymphoblastic blast with smooth chromatin and nucleoli present at 100x.
Bone marrow aspirate showing lymphoblastic blasts 40x (Wright-Giemsa stain).
Lymphoblastic blasts seen at 40x.
Bone marrow aspirate showing lymphoblastic blasts at 100x (Wright-Giemsa staining).
His karyotype: 46, XY, der (1) t (1;12) (p36;p13.2) [6]; 47, XY, der (1) t (1;12) (p36;p13.2) + 1 [4]; 47, XY, der (1) t (1;12) (p36;p13.2), + mar [7]. No BCR-ABL or MLL rearrangements were detected (by PCR analysis). There was no central nervous system involvement at the time of diagnosis. The patient’s evolution is shown in Table 1.
After diagnosis, treatment was started with Hyper-CVAD: hyper-fractioned cyclophosphamide, vincristine sulfate, doxorubicin hydrochloride (Adriamycin), and dexamethasone, two cycles. He did not respond, so a new induction regimen was performed with clofarabine, etoposide, and cyclophosphamide; however, the patient was again refractory. He then received FLAG-IDA (fludarabine, cytarabine, granulocyte colony-stimulating factor, and idarubicin) and remained refractory, with post-treatment bone marrow displaying 59.2% blasts. We then administrated a course of Nelarabine Peg-Asp AdmVP: doxorubicin 30 mg/m2 on days 1, 2, 3, 8; vincristine 2 mg/m2 on days 1, 8, 15, 22; pegylated-asparaginase 2500 U/m2 on days 15 and 22; prednisone 1 mg/kg from days 1 to 28, and nelarabine 1500 mg/m2 on days 22, 24 and 28. Triple intrathecal prophylactic chemotherapy with methotrexate 12 mg, cytarabine 100 mg, and dexamethasone 4 mg was performed. Before starting this course, we detected 0.06% blasts in cerebrospinal fluid (CD34, CD7, and CD3 +). Weekly intrathecal chemotherapy was performed until the cerebrospinal fluid was negative for blasts. He developed intraventricular catheter dysfunction, and it had to be removed. After the Nelarabine Peg-Asp AdmVP course on day + 35, minimal residual disease analysis showed no detectable blasts. His evolution was complicated by a bilateral grade-2 sensory-motor neuropathy probably related to nelarabine. According to the World Health Organization grading scale [8], a grade-2 corresponds to severe paresthesia and/or mild weakness. Symptomatology was bilateral pain and numbness from the knee to the ankle, and difficulty flexing knees. He received pregabalin 150 mg/day and kinesiotherapy. In addition, the patient developed a fever, with a computed tomography scan showing bilateral pulmonary nodules, and serum galactomannan levels of 0.04 (< 0.5 negative). He was initially treated with caspofungin and voriconazole before entering the conditioning phase of the transplant. A fully matched allogeneic stem cell transplantation from a sibling donor was performed. Conditioning consisted of fludarabine (30 mg/m2 for three days: -7, -6, and -5); Total body irradiation (200 cGy for three days: -4, -3, and -2). The patient received 3.3 x 106 CD34 + cells/kg without any manipulation. Graft-versus-host disease prophylaxis was performed with cyclophosphamide (50 mg/kg for two days: +3, and +4), tacrolimus, and mycophenolate. Infection prophylaxis included acyclovir, fluconazole, and levofloxacin. Granulocyte colony-stimulating factor (5μg/kg/day) was used from day +5 to +13. Absolute neutrophil count > 0.5 x 109/l and platelet count > 50 x 109/l took place on days +14 and +20, respectively. A skin rash was observed, and a skin biopsy showed lichenoid and spongiotic dermatitis; graft-versus-host disease was ruled out. The post-transplantation course was complicated by worsening fungal infection and progression of the previously identified pulmonary nodules. Bronchoalveolar lavage was performed without the identification of an infectious etiology. Serum galactomannan and beta-D-glucan were positive. He completed 14 days of liposomal amphotericin and voriconazole. After day +30, following the allogeneic stem cell transplantation, the patient had no detectable blasts and was discharged. Bone marrow assessment for minimal residual disease at day +100 was negative. He developed mild acute hepatic graft-versus-host disease in the third month after allogeneic stem cell transplantation, with a good response to an increased dose of tacrolimus. At the time of submission of this case report, the patient is in month 23 after allogeneic stem cell transplantation and remains in complete remission and resolution of his neuropathy. Notice that written informed consent was obtained from the patient for the publication of this case report.
Discussion
Nelarabine has been previously used in combination with the multi-drug regimens Hyper-CVAD and BFM86 [6,9,10]. Among 40 untreated or minimally treated patients (with no more than one induction course) treated in an MD Anderson Center study, 97% achieved a response, reaching 91% with a complete response. Poor outcomes were seen in patients with early T-cell precursor ALL compared to another T-cell ALL phenotype; 6 out of 11 patients with early T-cell precursor ALL failed to achieve a complete response, and the median overall survival was 22 months.
Compared to our cases, patients were treated with a lower dose of nelarabine (650 mg/m2 over five days versus 1500 mg/m2 over three days on our protocol). In other studies with refractory or relapsed pediatric patients, lower-dose nelarabine was combined with etoposide and cyclophosphamide [7] or etoposide and fludarabine in another study [11]. Response rates were three out of six, and five out of seven patients respectively. We chose a higher dose due to the high risk of our patient, not only because of the T-cell blasts type but also because he had failed to achieve induction with three different chemotherapy regimens. We chose the Nelarabine Peg-Asp AdmVP regimen since the patient had not received asparaginase before. The original Japanese protocol includes L-asparaginase [5], but we used pegylated-asparaginase because of its lower cost and greater gain in quality-adjusted life year [12]. The original study of this regimen demonstrated that 64% of patients achieved complete remission and single-agent response rates for nelarabine from 14 to 55% [13]. The BFM86 protocol included these drugs in the study [10], but that is part of a first-line pediatric protocol in which stem cell transplantation is not considered for the consolidation phase. Additionally, the dose of nelarabine was lower. To our knowledge, no studies have described this combination in refractory or relapsed adult patients.
Neurotoxicity is one of the most challenging toxicities associated with nelarabine, occurring in 21% of patients receiving the recommended dose of 1500 mg/m2 [14]. Neurotoxicity is a dose-dependent side effect, with grades three, or four being rare. The risk increases in multi-drug regimens. Our patient had been receiving vincristine in the first Hyper-CVAD course and again with the Peg-Asp AdmVP course. He developed neurological symptoms in his upper and lower limbs. He was diagnosed with grade-2 neuropathy. Our patient had no central nervous system affection, which is very rare and described only in two cases [4]. Our patient resolved his neurological symptoms one year after allogeneic stem cell transplantation.
Regarding stem cell transplantation, the overall survival rate after the nelarabine treatment ranges from 29 to 40%, and transplantation-related mortality from 11 to 13% [3]. According to Cardonni et al. [3], patients with responsive disease should be allografted as soon as possible, regardless of sibling donor availability. Our patient was minimal residual disease negative after chemotherapy with Nelarabine Peg-Asp AdmVP and only eight days later was undergoing conditioning for an allogeneic stem cell transplantation.
Conclusions
While more clinical trials are needed, the Nelarabine Peg-Asp AdmVP regimen seems effective and safe. Further research needs to be carried on, so that this could be established as an induction treatment in refractory early T-cell precursor acute lymphoblastic leukemia.

