Reporte de caso
← vista completaPublicado el 10 de marzo de 2023 | http://doi.org/10.5867/medwave.2023.02.2674
Úlcera vulvar aguda de Lipschütz relacionada con la vacunación por COVID-19: primer caso notificado en Sudamérica
Lipschütz acute vulvar ulcer related to COVID-19 vaccination: First case report in South America
Abstract
Lipschütz ulcer is a non-sexually transmitted genital lesion of unknown etiology, which presents as a painful vulvar ulcer. Lipschütz ulcers have been described in most continents. This is the first case reported in Peru and South America. We present the case of a 33-year-old female patient with a Lipschütz ulcer after being vaccinated with the second dose of the AstraZeneca COVID-19 vaccine. She reported having had only one sexual partner in her lifetime. Laboratory results were negative for herpes simplex 2, Cytomegalovirus, Toxoplasma gondii, Epstein-Barr virus, and syphilis. The patient received symptomatic treatment. Ten days after the onset, the patient was significantly better during follow-up. This case report displays a potential adverse effect of the AstraZeneca COVID-19 vaccine as a Lipschütz ulcer triggered by the host humoral immune response. However, further research is needed to establish the causal relationship between these two.
Main messages
- Lipschütz ulcers are non-sexually transmitted genital lesions of unknown etiology, which present as painful vulvar ulcers.
- This is the first case reported in Peru and South America.
- The diagnosis is by exclusion, the main differential diagnoses being sexually transmitted infections and certain systemic diseases.
- This case report informs the appearance of Lipschütz ulcers after the patient’s second dose of the AstraZeneca COVID-19 vaccine, suggesting that the vaccine triggers the humoral immune response.
Introduction
Lipschütz ulcers, also known as ulcus vulvae acutum or vulvar aphthous ulcers, were first described in 1913 by an Australian dermatologist, Dr. Benjamin Lipschütz [1,2]. They are mainly seen in sexually inactive young girls with no specific cause found. These non-sexually transmitted genital ulcers have an acute onset as painful ulcers on the vulva [3].
The etiology remains unknown, and they have been associated over the years with different viral infections such as Epstein-Barr virus, cytomegalovirus, and influenza A, among others [4]; however, this theory has not been proven.
Lipschütz ulcers usually evolve favorably and go into spontaneous remission [5]; even so, they are quite painful and can cause considerable discomfort in patients.
This case report presents a 33-year-old female patient with a Lipschütz ulcer after being vaccinated with the AstraZeneca COVID-19 vaccine. This is the first case reported in Peru and South America.
Case presentation
A 33-year-old woman attended the gynecology emergency department of a high-complexity Peruvian private clinic with excruciating vulvar pain and malaise. The previous week, she had been to the outpatient clinic for a suspected vulvar genital ulcer due to herpes simplex vulvar, received topical treatment with acyclovir, and was sent home. Three days later, she returned to the outpatient clinic because of persistent vulvar pain, rated 10 out of 10 on the analog visual scale. She was prescribed oral antibiotics and nonsteroidal anti-inflammatory drugs and sent home. The pain and malaise persisted throughout the days.
Her medical history indicated that the patient received the second dose of the AstraZeneca COVID-19 vaccine three days before the onset of the ulcers. She reported having had only one sexual partner during her lifetime, denying any recent sexual or genital contact. She worked as a teacher and denied any toxicological history. There was a history of allergic rhinitis, polycystic ovary, and COVID-19 two months earlier. Oral contraceptives and antihistamines were frequently used drugs.
Physical examination stated: blood pressure: 120/70 mmHg, heart rate: 78 beats per minute, and respiratory rate: 16 breaths per minute, afebrile. The patient appeared to be in significant pain.
Genital examination revealed an ulcer of approximately 4 × 4 cm with indurated and well-defined edges, medial location on the left labia minora, erythema, edema (Figure 1), and intense discomfort during the examination.
Initial presentation of the vulvar ulceration: 4 × 4 cm ulcer on the inner face of the left labia minora at 1 cm from the vaginal fourchette; painful and deep, with local edema and covered with fibrinoid material.
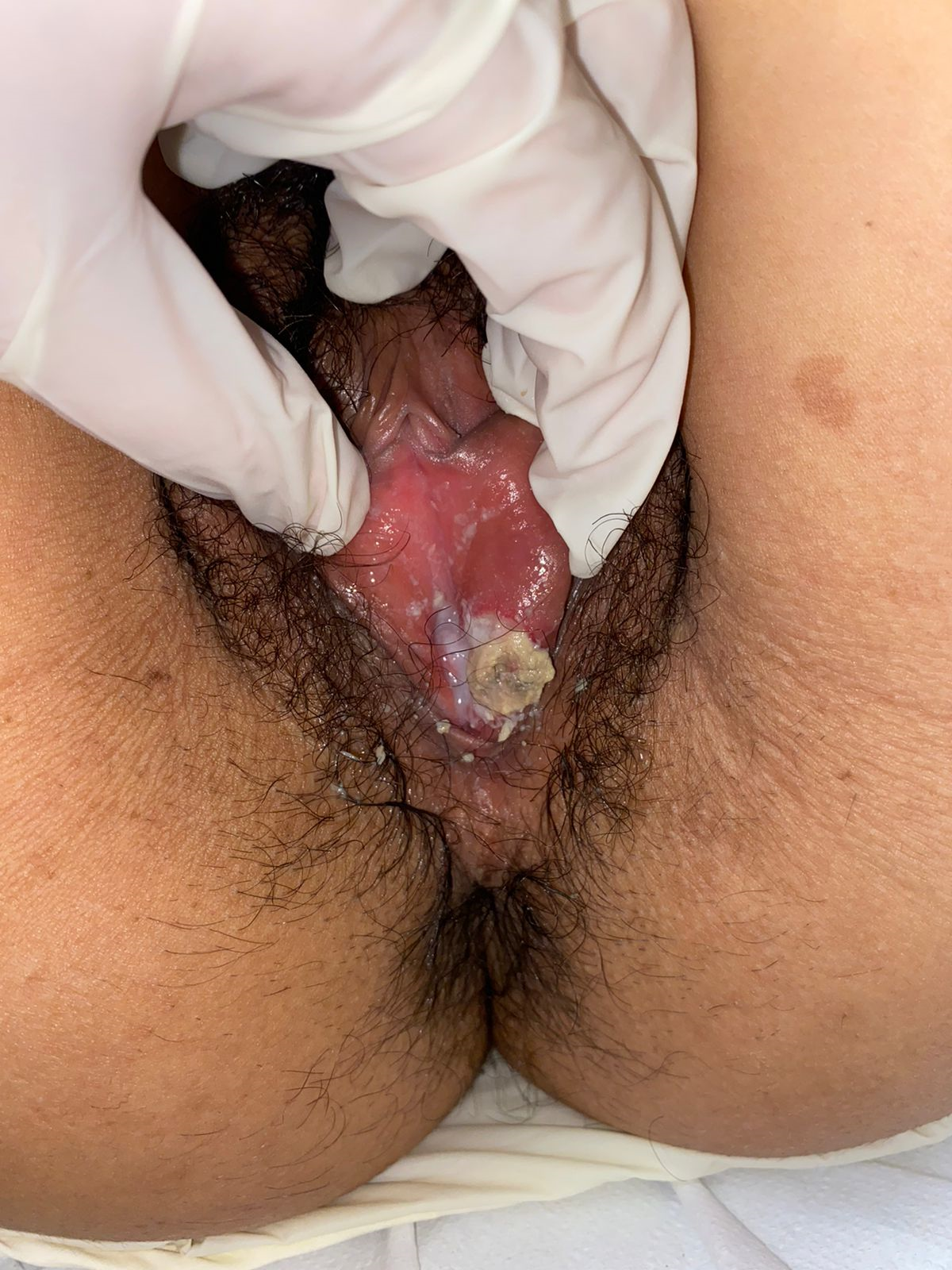
Oropharyngeal examination revealed two oral aphthae on the right side of the buccal mucosa. The remainder of the examination revealed no further findings.
The symptoms were relieved by administering 1 g intravenous ketoprofen and 4 mg of dexamethasone at the emergency room after she was discharged with 100 mg of ketoprofen three times a day for five days, 20 mg of prednisone daily for seven days, and 500 mg of cefuroxime twice a day for seven days due to suspected bacterial etiology. Laboratory results: IgM and IgG antibodies against herpes simplex virus 2 were negative, and IgM against Cytomegalovirus, Toxoplasma gondii, and Epstein-Barr virus were negative. Syphilis serology (rapid plasma reagin) and culture of vaginal secretions were also negative. Clinical history, physical examination, and laboratory evaluation were consistent with a vulvar aphthous ulcer following the AstraZeneca-COVID-19 vaccination.
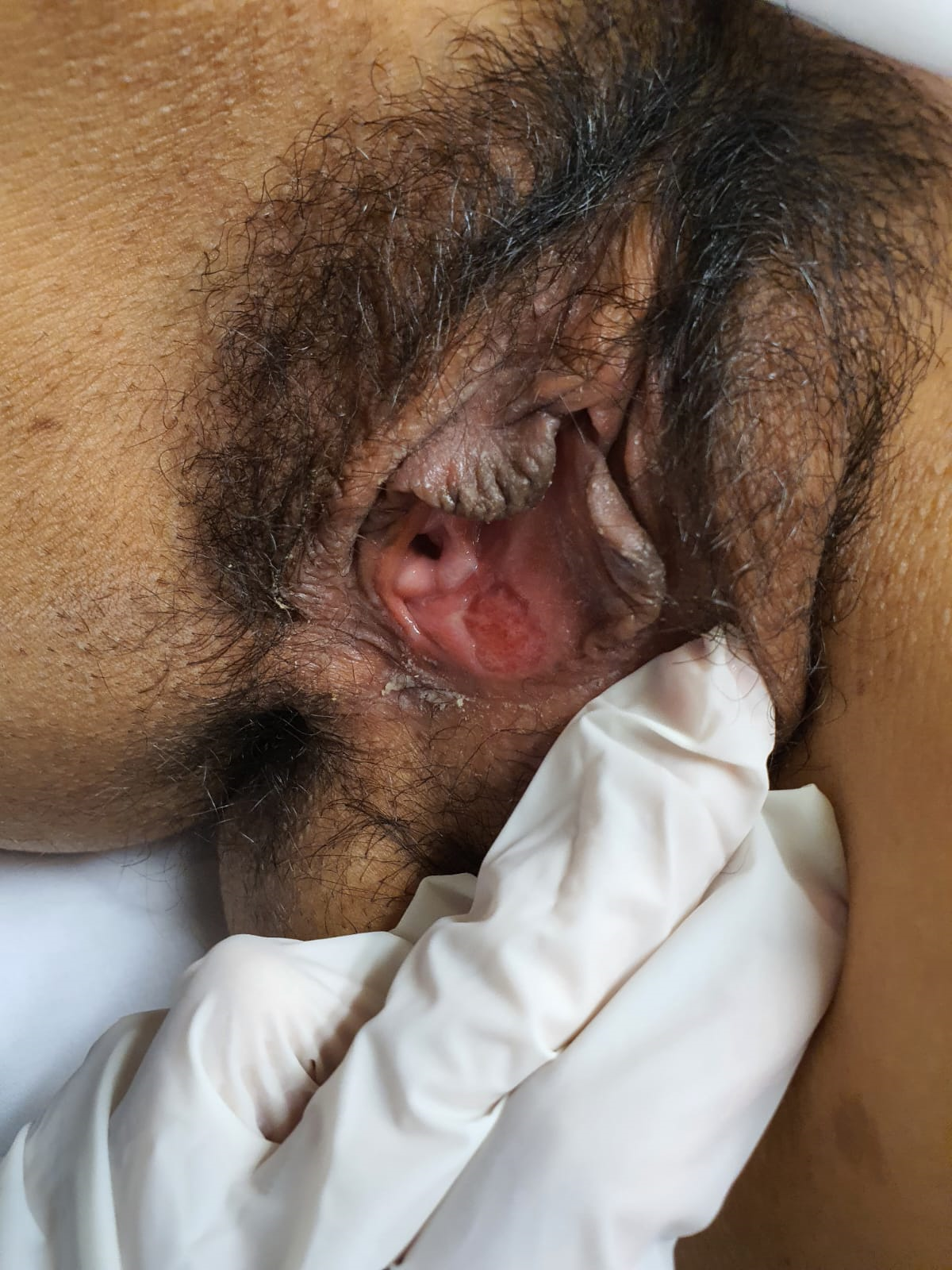
During the follow-up, ten days after the initial presentation, the patient improved significantly with the prescribed medication, with no pain; no edema or erythema, nor secretions were observed, and there was a decrease in the size of the ulcer (Figure 2). Subsequently, after two weeks, the patient reported completing the treatment without any pharmacological adverse events and began to feel better progressively. Examination revealed a complete resolution of the ulcer and symptoms.
Follow-up image 10 days after initial presentation and one week of medical treatment with NSAIDs and corticosteroids.
A shallow 3 × 2 cm ulcer with well-defined edges, without edema, fibrinoid tissue, or signs of phlogosis.
Discussion
Vulvar ulcers are usually caused by different etiologies such as syphilis, lymphogranuloma venereum, chancroid, granuloma inguinale, and herpes simplex virus. Less frequently, vulvar ulcers have a non-infectious etiology, such as drug reactions, autoimmune diseases, trauma, or aphthous ulcers [3].
Aphthous ulcers, known as Lipschütz ulcers, have been described in almost all continents [4,5,6,7,8,9]. Their incidence is unknown, but according to the WHO global database on reported potential side effects of medicinal products, 139 cases of vaginal and 27 cases of vulvovaginal ulcerations were associated with the COVID-19 vaccines since the beginning of the pandemic [10].
Although the etiology is still unknown, they have been associated with infectious diseases and inflammatory and autoimmune conditions. Viruses have been the most frequently associated factor with these ulcers [11], especially Epstein Barr virus, CMV, influenza, and recently SARS-CoV2 [11]. The latter is related to its infectious process and is a possible cause of adverse reactions to its vaccines.
Pfizer-BioNTech and AstraZeneca-COVID-19 vaccines, according to their mechanism of action, trigger the exposure of SARS-CoV2 virus particles to the immune system, reducing the risk of severe episodes and mortality on subsequent natural exposure to them [11]. By releasing RNA through lipid nanoparticles into the host cell, the Pfizer-BioNTech vaccine triggers S-antigen expression and can activate the immune response [12]. The AstraZeneca-Covid-19 vaccine is a monovalent vaccine composed of a recombinant chimpanzee adenovirus vector, which encodes the S-glycoprotein of the SARS-CoV2 virus capable of triggering the desired humoral and cellular response [13]. It is administered in two intramuscular injections, 4 to 12 weeks apart. Different vaccine reactions have been described in the subcutaneous tissue, such as hyperhidrosis, pruritus, rash, and urticaria [13]. As the COVID-19 pandemic endures and COVID-19 vaccination continues, new potential adverse effects arise.
Since 2021, several cases of Lipschütz ulcer have been reported in the USA following the Pfizer-BioNTech COVID-19, most of the cases after the second dose of the vaccine and in the pediatric population [6,7].
In Spain, four cases of Lipschütz ulcers were reported after vaccination with AstraZeneca COVID-19, considered vaccine adverse reactions, mainly reported in adults [8].
These reports have similarities with the one we present, as they were also triggered by the second dose of the COVID-19 vaccine, following the hypothesis that previous exposure to SARS-CoV-2 virus particles and certain unknown characteristics of the individual could cause Lipschütz acute vulvar ulcer [11].
Diagnosis is by exclusion, with differential diagnoses being mainly sexually transmitted diseases and systemic diseases such as Crohn’s, Behcet’s, fixed drug eruption, and neoplasia [9]. Since its first description by Lipschütz, few case reports and case series have been published, so it may be underdiagnosed [9]. Its clinical features are the basis for diagnosis. It typically presents as up to three deep ulcers larger than 10 mm, with the following: hyperalgesia, well-defined edges, necrotic, fibrinous, or purulent base, mirror-like vulvar distribution, and acute evolution [4]. They usually appear in the vestibule and labia minora [7], are associated with regional lymphadenopathy, often preceded by a flu-like syndrome [8], and tend to resolve in less than three weeks when associated with a non-venereal infection. Sadoghi [9] proposed two major diagnostic criteria: acute onset of one or more painful ulcerative lesions in the vulvar region and the exclusion of infectious and other non-infectious causes. He also proposed minor criteria: location of the ulcer in the vestibule or labia minora, absence of sexual intercourse in the last three months, flu-like symptoms, and systemic infection within two to four weeks prior to the appearance of the vulvar ulcer. The diagnosis is established if the two major and at least two minor criteria are present. In our case, the criteria are met; therefore, the diagnosis is justified.
All those etiologies had been excluded in this case report, suggesting that the vulvar ulcer could be a humoral immune response to the COVID-19 vaccine due to virus exposure. Additionally, she responded well to symptomatic treatment with NSAIDs and corticosteroids, having a favorable resolution.
Recommended treatment is based on pain management, local hygiene, wound care, and relief of associated symptoms. Since corticosteroid treatment may prolong the clinical course, it is only suggested in severe cases [4,9]. Sadoghi and collaborators [9] suggested treatment options depending on the clinical presentation of the vulvar ulcer; topical anesthetics and NSAIDs for mild to moderate and/or severe (highly inflamed) cases; the latter can also be treated with topical and systemic corticosteroids. In addition, if the vulvar ulcer presented with concomitant nonspecific symptoms such as malaise, fever, and phlogosis, he suggested dosing inflammatory markers and using broad-spectrum antibiotics if needed.
The presented case was treated as a severe ulcer with concomitant nonspecific symptoms, using topical and systemic corticosteroids, antibiotics, and NSAIDs.
Reporting potential adverse effects of COVID-19 vaccines will help physicians and scientists learn more about them, and patients will have more peace of mind and continue to be vaccinated, contributing to better care.
Conclusion
In summary, Lipschütz acute vulvar ulcer has no known etiology and presents acutely and painfully. Resolution is spontaneous, but local hygiene, wound care, and symptom control are the major pillars of treatment.
This case report exposes a potential adverse effect of the AstraZeneca-COVID-19 vaccine in the form of a Lipschütz acute vulvar ulcer that triggered the adult host humoral immune response to the vaccine. However, further research is needed to establish the causal relationship between these two.

