Epistemonikos summaries
← vista completaPublished on May 7, 2016 | http://doi.org/10.5867/medwave.2016.6438
Is racecadotril effective for acute diarrhea in children? -First update
¿Es efectivo el racecadotrilo como tratamiento para la diarrea aguda en niños? -Primera actualización
Abstract
This article updates the December 2015 Living FRISBEE (Living FRISBEE: Living FRIendly Summary of the Body of Evidence using Epistemonikos), based on the detection of two systematic reviews not identified in the previous version.
Gastroenteritis or acute watery diarrhea is usually a self-limited disease, but it is still associated to substantial healthcare costs and remains a frequent demand for medical care. Racecadotril, an intestinal enkephalinase inhibitor, has been used as treatment because it would decrease the duration of acute diarrhea and fluid loss. However there is still no evidence supporting its routine use. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified five systematic reviews including nine randomized trials relevant for our question. We combined the evidence using meta-analysis and generated a summary of findings table following the GRADE approach. We concluded racecadotril probably reduces the duration of acute diarrhea in pediatric patients, without increasing adverse effects.
About the update
This article is an update of the Living FRISBEE (Living FRISBEE: Living FRIendly Summary of the Body of Evidence using Epistemonikos) published in December 2015 (doi: 10.5867/medwave.2015.6339), based on two systematic reviews not identified in the previous version [1],[2]. One of them [1] includes a new randomized controlled trial not answering our question because it compares racecadotril with loperamide, not placebo. The same systematic review [1] contributes with a new meta-analysis on adverse effects of racecadotril.
The new evidence incorporated in this summary does not lead to changes in the certainty of the evidence or magnitude of the effects. One systematic review incorporated a new meta-analysis adding new issues to the considerations for decision-making [1].
Problem
Gastroenteritis or acute watery diarrhea is usually a self-limited disease, lasting five to seven days. However, it is still associated to substantial healthcare costs and remains a frequent demand for medical care. The main complication in pediatric population is severe dehydration. Therefore, the main aim of treatment is to prevent it; oral rehydration solution is the cornerstone of therapy, but it does not decrease the duration of diarrhea or fluid loss.
Racecadotril is an antisecretory drug that prolongs endogenous enkephalin action by inhibiting intestinal enkephalinase, decreasing the secretion of water and electrolytes. Even though the mechanism of action seems promising, there is still no evidence to support its routine use for acute diarrhea in children.
Methods
We used Epistemonikos database, which is maintained by screening more than 30 databases, to identify systematic reviews and their included primary studies. With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found five systematic reviews [1],[2],[3],[4],[5] including ten randomized controlled trials, reported in nine references [6],[7],[8],[9],[10],[11],[12],[13],[14] (one reference reports two studies [12]). One of the trials [14] compares racecadotril with loperamide, so we did not include it in the analysis. |
|
What types of patients were included |
All trials restricted inclusion to pediatric population, between 3 to 71 months old, with acute diarrhea of viral or bacterial etiology. Four studies considered inpatients only [6],[7],[10],[12] and five outpatients only [8],[9],[11],[12],[13]. Stool output and number of diarrheic stools per day before inclusion were registered, showing no difference between racecadotril and placebo groups. |
|
What types of interventions were included |
Trials evaluated oral racecadotril; in six the dose was 1.5 mg/kg [6],[7],[8],[9],[10],[11], two administered 10 mg or 20 mg three times a day adjusted by body weight (over or under 9 kg) [12] and one did not specify dosage [13] All studies compared adding racecadotril to standard treatment (oral rehydration solution). Three studies were placebo controlled [6],[7],[10]. |
|
What types of outcomes |
Proportion of responders (defined as diarrhea duration less than 48 hours), proportion of patients not cured at fifth day of therapy, daily stool output and volume, number of vomits the first 48 hours of treatment and adverse effects reported during treatment. |
Summary of findings
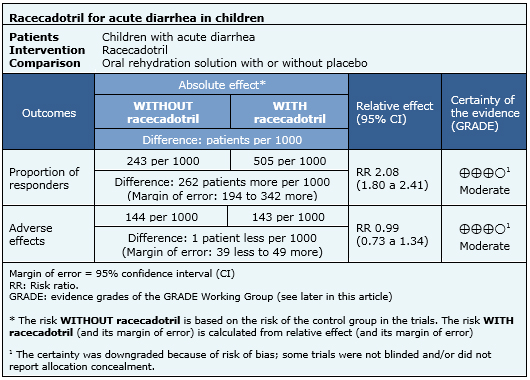
The following information about the effects of racecadotril is based on nine randomized trials that included 1384 patients. Our analysis is based on the systematic review that conducted an individual patient data analysis [5]. The outcome measured was resolution of acute diarrhea before 48 hours of treatment. Giving the relevance for decision making, this summary considers the meta-analysis of five randomized controlled trials [6],[7],[8],[12] reported in one systematic review [1] that includes data about adverse effects reported during therapy with racecadotril.
- Racecadotril probably reduces the duration of acute diarrhea in pediatric patients. The certainty of the evidence is moderate.
- Racecadotril probably does not increase adverse effects. The certainty of the evidence is moderate.
Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
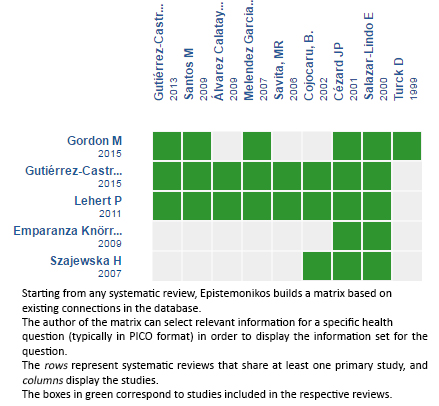
Follow the link to access the interactive matrix of Evidence. Racecadotril for acute diarrhea in children
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

