Short communications
← vista completaPublished on September 16, 2020 | http://doi.org/10.5867/medwave.2020.08.8027
Use of Cochrane reviews in nationally-developed clinical practice guidelines in Latin America
Uso de revisiones Cochrane en guías de práctica clínica desarrolladas a nivel nacional en América Latina
Abstract
Introduction Cochrane reviews, recognized as the benchmark for high-quality summaries, facilitates healthcare decision-making bringing together all the evidence on an intervention. To date, their inclusion in the Latin American guidelines remains unknown.
Objective To evaluate the use of Cochrane reviews in nationally-developed clinical practice guidelines in Latin America.
Methods We conducted a hand search in official government websites and biomedical databases between October 2019 and December 2019, including government-sponsored clinical practice guidelines with recommendations for both the management of health conditions or a healthy lifestyle of the last ten years.
Results We included 408 clinical practice guidelines from ten countries. We found that 69.8% of them cited Cochrane reviews in their recommendations, and 76.1% of those also used them in their key recommendations. Clinical practice guidelines that did not use Cochrane reviews covered a wide range of topics for which several Cochrane reviews can be found. Countries using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach for grading recommendations were more likely to use Cochrane reviews in a higher percentage of their guidelines (79.4% vs. 61.8%; odds ratio: 2.3; 95% confidence interval: 1.5 to 3.7, p = 0.0001).
Conclusions Over two-thirds of clinical practice guidelines in Latin America use Cochrane reviews to frame their recommendations. It is necessary to increase the uptake of Cochrane reviews in the region for the development of clinical practice guidelines.
|
Main messages
|
Introduction
Cochrane’s primary aim is to help people make well-informed decisions about healthcare by preparing, maintaining and promoting the accessibility to systematic reviews of the evidence. Cochrane’s work is internationally recognized as the benchmark for high-quality information about the effectiveness of health care, as determined by the evaluation of methods used to minimize bias within a study design and the statistical precision of the measured effects, among other criteria. By providing a reliable synthesis of the available evidence on a topic, systematic reviews adhere to the principle that science is cumulative and facilitates decisions while considering all the evidence on the effect of an intervention[1]. Clinical practice guidelines are one of the most useful tools for improving clinical practice and public health, as they offer information for interventions taking into account the balance between benefits and harms and the use of resources, reducing clinical variability, improving health, and ensuring the quality of care[2]. In the framework of Cochrane’s Strategy 2020[3] for producing evidence, making the evidence accessible, advocating for evidence and building an effective sustainable organization to put Cochrane’s evidence at the heart of health decision-making, and to make Cochrane the ‘home of evidence’ to inform health decision-making across the world, including lower and middle-income and non-English speaking countries such as those in the Latin American region, it would be reasonable for clinical practice guidelines development groups to base their recommendations on Cochrane reviews or to moderately interact with the reviews’ development team[4]. But to date, their contribution to clinical practice guidelines in Latin America remains unknown. In this region, nationally-developed clinical practice guidelines are widely used by health care practitioners, although high-quality evidence-informed clinical practice guidelines are still lacking in most countries[5]. The dissemination of Cochrane’s reviews could allow health care professionals and patients in Latin America access to evidence for adequate decision-making[6]. The description of the overall and/or topic-specific lack of uptake of Cochrane reviews in clinical practice guidelines might help Cochrane prioritize the production and dissemination of its evidence along the region.
Methods
We conducted a comprehensive hand search in official government websites and biomedical databases (MEDLINE, EMBASE, and LILACS) between October 2019 and December 2019, including government-sponsored clinical practice guidelines with recommendations for the management of health conditions or recommendations for a healthy lifestyle within the last 10 years. The aim was to evaluate the use of Cochrane reviews in nationally-developed clinical practice guidelines in Latin America. We retrieved documents identified as clinical practice guidelines, including a method for guideline development, regardless how succinct they were. Whenever we found several versions of the same clinical practice guideline, we included the latest version of the updated guideline. For consistency of criteria, we first conducted a pilot test of duplicate independent data extraction with the first 50 included clinical practice guidelines. The extraction of the remaining guidelines was done later by a single author. Cochrane reviews were identified by inspecting references or by inspecting the guideline document in their methods or search strategy section. When a Cochrane review was identified, we also verified if it supported recommendations and key recommendations. We reported continuous variables as mean and standard deviation (SD) or as median and range according to distribution. We reported categorical variables as proportions. We explored the association between Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach use in guideline methods and Cochrane reviews’ use estimating the odds ratio using STATA software.
Results
We identified 2,828 records through database searching and hand search. After duplicate removal, 2,807 records were assessed for eligibility. We excluded 1,148 records due to lack of methods section, 259 records due to development by scientific societies or social security agencies, 206 records due to publishing dates before 2009, 125 records that were classified as protocols, programs or norms and 661 due to other causes. We finally included 408 clinical practice guidelines from ten countries (Figure 1).
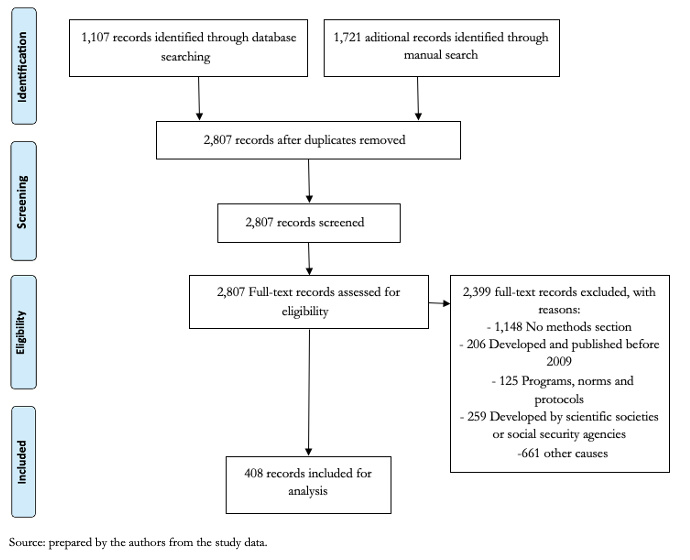 Full size
Full size We found that 69.8% of records cited Cochrane reviews in their recommendations and 76.1% of those also used them in their key recommendations (Table 1). Clinical practice guidelines citing Cochrane reviews in their recommendations ranged from 66% of guidelines in Argentina to 100% of guidelines in Peru and Honduras. Using Cochrane reviews to frame key recommendations ranged from 22% of guidelines in Argentina to 100% of guidelines in Peru and Honduras. We also found that topics of the guidelines in which no Cochrane reviews were cited in the recommendations were highly variable: chronic kidney disease, diabetes mellitus, celiac disease, Chagas disease, breast cancer screening, retinopathy of prematurity, scoliosis, arrhythmias, primary tumors of the central nervous system, and cervical cancer. Additionally, only 45.3% (n = 185) of clinical practice guidelines used the GRADE approach[5] for grading their recommendations, ranging from 13.6% of guidelines in Brazil to 88.8% of guidelines in Colombia. Aside from Brazil and Ecuador that used Cochrane reviews in a high proportion of guidelines (68% and 70%, respectively) but were less keen to use the GRADE approach for grading guidelines recommendations (13% and 11%, respectively), countries using the GRADE approach for grading recommendations were more likely to use Cochrane reviews in a higher percentage of their guidelines (79.4% vs 61.8%; odds ratio: 2.3; 95% confidence interval: 1.5 to 3.7, p = 0.0001) (Table 2).
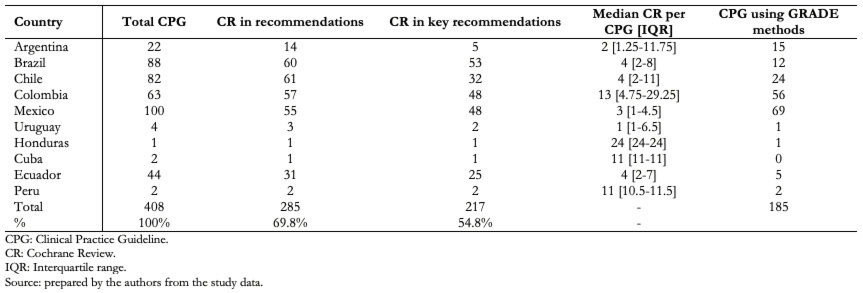 Full size
Full size  Full size
Full size Discussion
There are several explanations to our findings. For some specific topics, there may not be evidence available due to lack of randomized controlled trials to be included in systematic reviews. Alternatively, there might not be an up-to-date Cochrane review including relevant trials, which would run in opposition to the GOAL 1 of Cochrane’s Strategy 2020[3] for review production and update. There may also be an inadequate dissemination of Cochrane evidence in some countries in Latin America, opposing the GOAL 3 of Cochrane’s Strategy 2020 for knowledge translation and the Iberoamerican Cochrane Centre dissemination activities[7], and which could impede their uptake among healthcare professionals and clinical practice guideline developers. This is in contrast to the use of Cochrane reviews in the World Health Organization guidelines, which was reported to be 90% in 2016[8]. The poor report and quality found in the included guidelines, like those with a missing reference section or reference section not numbered, may not allow the proper identification of Cochrane reviews and their use to frame key recommendations.
Our research has several limitations. First, the search method included a high proportion of hand search. This was due to the small number of government-sponsored clinical practice guidelines that usually follow a formal editorial process in scientific journals and biomedical databases. Also, clinical practice guidelines storage in government websites was highly variable across countries and many links to their full versions were broken. Lastly, although it was not the aim of our research, we were not able to thoroughly explore the reasons for the lack of uptake of Cochrane reviews. The description of the overall and/or topic-specific lack of uptake of Cochrane reviews in clinical practice guidelines might help Cochrane to prioritize the production and dissemination of its evidence along the region.
Regardless, we made a comprehensive and integral approach on Cochrane reviews uptake in clinical practice guidelines throughout Latin America, a region with a wide heterogeneity in health care systems and economic resources for research or clinical practice guidelines development. We also included a high number of guidelines in both Spanish and Portuguese and made an exhaustive literature search to identify all available clinical practice guidelines. Included clinical practice guidelines were rigorously appraised by highly trained researchers with vast experience in methods and evidence-based medicine.
Conclusions
Over two thirds of clinical practice guidelines in Latin America use Cochrane reviews to frame their recommendations. However, a significant proportion of clinical practice guidelines in Latin America still do not use Cochrane reviews to frame their recommendations. These guidelines covered a wide range of topics; there might be up-to-date Cochrane evidence for some of these topics. These findings highlight the need to disseminate the results of current Cochrane reviews and the need to identify topics in which updated Cochrane reviews are not available.
Notes
Authorship contributions
LG, PR, JVAF: Conceptualization, methodology, formal analysis, investigation, writing (original draft preparation, review and editing). CEL, NM, MA, EM: Methodology, investigation, writing (original draft preparation, review and editing).
Competing interests
The authors declare that they have no conflicts of interest involving this work.
Funding
The authors declare that there were no external sources of funding.
Ethics
This study did not require evaluation by an ethical–scientific committee because it is based on secondary sources.
From the editors
The authors submitted this article simultaneously in Spanish and English. Both version have been copyedited by the journal.

