Research papers
← vista completaPublished on June 12, 2018 | http://doi.org/10.5867/medwave.2018.03.7211
Observational study to analyze patterns of treatment of breakthrough dyspnea in cancer patients in clinical practice
Estudio observacional para analizar las pautas de tratamiento de la disnea irruptiva en pacientes con cáncer en la práctica clínica
Abstract
INTRODUCTION Although breakthrough dyspnea is very frequent in cancer patients, there are no precise recommendations for treating it. The main objective of this study was to analyze what treatments are used in clinical practice for the management of breakthrough dyspnea in cancer patients in Spain and the secondary objectives were to describe the characteristics of cancer patients with breakthrough dyspnea and the attributes of the disorder.
METHODS Cancer patients over 18 years of age, with breakthrough dyspnea and a Karnofsky performance score of ≥30, who were treated at departments of oncology in institutes across Spain were included in this cross-sectional observational study. The characteristics of breakthrough dyspnea, history of treatment, anthropometric variables, Mahler dyspnea index, Borg scale, Edmonton Symptoms Assessment Scale, and patient satisfaction with current breakthrough dyspnea treatment were assessed.
RESULTS The mean age of the 149 included patients was 66 years (95% confidence interval: 64.3 to 67.9), and 53 were females (35.6%). The mean breakthrough dyspnea intensity was 5.85 (95% confidence interval 5.48 to 6.22, Borg scale). A total of 55.1% of the first-choice treatments consisted of opioids, followed by oxygen (17.3%). A total of 119 patients (79.9%) received monotherapy for breakthrough dyspnea. Patients presenting with basal dyspnea received oxygen in a greater proportion of cases (21.1% vs 7.4%; p = 0.07). Patients with predictable dyspnea received a greater proportion of opioids (70.9% vs 44.4%; p = 0.01).
CONCLUSIONS Opioids constitute first-line therapy for breakthrough dyspnea in routine clinical practice, though the scientific evidence supporting their use is scarce. Further information derived from controlled clinical trials is needed regarding the comparative efficacy of the different treatments in order to justify their use.
Introduction
Dyspnea is one of the most common symptoms in oncology patients with advanced-stage disease. It is an unpleasant symptom with a strong impact on the patients, their relatives and their healthcare teams. Dyspnea is present in the evolution of many disorders, and its prevalence increases with disease progression and with physical deterioration of the affected patients.
Dyspnea can manifest in a continuous and progressive manner or in the form of crises. There is no consensus-based definition of what constitutes a crisis, also called breakthrough dyspnea due to its similarity with breakthrough pain. The prevalence of breakthrough dyspnea is therefore difficult to establish, due to this lack of definition, though it is estimated to affect 81-85% of cancer patients [1],[2].
Based on the data provided by Simon et al. in their 2013 review [3], breakthrough dyspnea can be defined as an episode of sudden-onset, acute breathing difficulty in a patient with or without background breathing problems that is self-limiting in time and with a duration of less than 10 minutes in most cases. The intensity of breakthrough dyspnea is ≥ 6 points on the Borg scale (very severe dyspnea). The condition usually manifests daily (1 to 5 episodes). Breakthrough dyspnea can be associated with prodromal physical or emotional sensation that alerts the patient, and is accompanied by other symptoms such as pain, cough and fatigue. Triggering factors such as physical exertion may intervene in some cases, though in other cases the patient is unable to identify any such factors [3].
Breakthrough dyspnea has a strong impact on the physical and psychosocial condition of the patient, and his/her quality of life [4],[5],[6],[7]. It also has a negative impact on the quality of life of the caregivers [1],[2],[3],[4],[8],[9],[10]. The management of breakthrough dyspnea usually depends on the experience of the healthcare team, the underlying etiopathogenic mechanism, the background disease, patient characteristics and location, prognosis, and the available resources. None of the strategies used, whether pharmacological or otherwise, are supported by scientific evidence of sufficient quality to be included in clinical practice guidelines [11].
To date, no study has conducted an in-depth investigation of the characteristics of breakthrough dyspnea or of the treatments used in this setting. Therefore, the main objective of this study was to analyze what treatments are used in clinical practice for the management of breakthrough dyspnea in cancer patients and the secondary objectives were to describe the characteristics of cancer patients with breakthrough dyspnea and the attributes of the disorder.
Methods
Study design and ethical standards
This was a cross-sectional observational study. Patients were included between May 2015 and March 2016. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Specialists from 23 Departments of Oncology in 16 Spanish provinces participated in the study. The investigators completed a case report form specifically designed for the study.
Screening criteria
Patient screening was performed on a consecutive random basis, selecting the first 10 outpatients visiting the clinic who met the inclusion criteria. The source documents were the clinical history and the data collected during the screening visit.
Patients of all races, both genders, aged over 18 years with a diagnosis of breakthrough dyspnea and oncological background disease were included. Patients with cognitive problems or who were severely affected by their background disease, with a Karnofsky performance score of <30, and uncooperative individuals, were excluded.
Breakthrough dyspnea was defined as sudden-onset, acute breathing difficulty in a patient with or without background of breathing problems that is self-limiting in time and with a duration of <10 minutes, with an intensity of ≥6 points on the Borg scale (very severe dyspnea).
Sociodemographic and clinical variables
The patient’s date of birth, gender, weight, height, hydration status (poor, average, good) at the time of consultation, and socioeconomic level (low: income less than 15,000 euros/year; average: >15,000-25,000 euros/year; high: >25,000 euros/year) were recorded. Functional status was assessed based on the Karnofsky performance score, from 0-100% [12]. Patient medical history was collected and the intensity of basal dyspnea was assessed using the Mahler basal dyspnea index. The Mahler index is an indirect multidimensional scale. It comprises three subscales that explore three components of dyspnea: task magnitude, functional disability and exertion magnitude. Each dimension is scored using a 5-point scale from 0 (intense) to 4 (none), and the total sum yields a global score of between 0 and 12. The lower the score, the greater the intensity of dyspnea [13]. In the case of patients with basal dyspnea, we recorded the date of onset of dyspnea. We recorded oxygen saturation at the time of the visit using pulsioxymetry (saturation percentage).
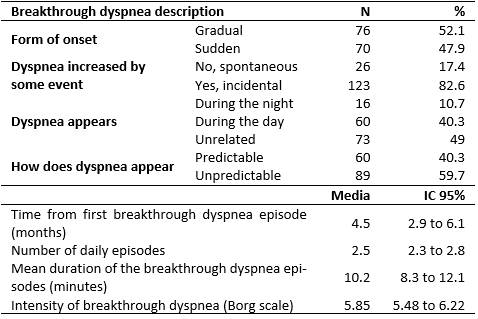
Information was collected on the main characteristics of breakthrough dyspnea: first episode or patient on follow-up; date of first breakthrough dyspnea episode; number of episodes per day or per week; form of onset (gradual or sudden); total duration of the episode; exacerbating factors (movement, cough, swallowing, bowel movements, others); manifestation at night or during the day, or with no relation to the time of day; and predictability (predictable or not predictable).
Clinical assessment
The patients were asked to report the average intensity of breakthrough dyspnea when it occurred, scoring the intensity with the Borg scale. The Borg scale is a unidimensional visual analogue scale (VAS) from 0-10 points, and presents descriptors associated with several categories [14]. The patients also recorded the intensity of dyspnea at the time of the visit using a 10-cm VAS, where 0 = “No breathing difficulty” and 10 = “Maximum breathing difficulty”.
The Edmonton Symptoms Assessment Scale (ESAS) was used to assess other associated symptoms that occurred in the preceding week: pain, tiredness, nausea, depression, anxiety, drowsiness, anorexia, malaise, resting dyspnea and insomnia [15].
Treatment
Some patients received medical treatment and/or care for their clinical condition according to the clinical judgment of the specialist. Information was collected on the history of consecutive non-pharmacological and pharmacological treatments administered for breakthrough dyspnea from the time of first onset of the condition, along with the medications used by the patient for other indications at the time of consultation. Patients scored their satisfaction with the treatment they received for breakthrough dyspnea at the time, using a 10-point VAS, where 0 = “Not at all satisfied” and 10 = “Completely satisfied”. Patients were considered to be satisfied with the treatment if the score was ≥5 points.
Statistical analysis
No formal sample size was calculated for this study.
A descriptive analysis was made of the variables included in the study, based on the distribution of frequencies for qualitative variables, calculation of percentages for qualitative variables, and calculation of the mean, standard deviation, minimum and maximum for quantitative variables. Comparisons between qualitative variables were made using the Fisher test or the Chi2 test (treatment with opioids by previous treatment with opioids, treatment with opioids by predictable or not predictable dyspnea). The Student t-test was used to compare independent groups in the case of quantitative variables (age by sex, body mass index by sex, Mahler score by sex, Borg scale by sex, intensity of basal dyspnea by sex). On evaluating differences in quantitative variables between groups with more than two categories, we performed factor analysis of variance applying Bonferroni or Games-Howell correction according to the homogeneity of variances to control for error in multiple comparisons. This analysis was applied to the comparison of number of daily episodes of breakthrough dyspnea by first choice of monotherapy treatment, and satisfaction of the patient with current treatment of breakthrough dyspnea.
The statistical significance level was set at 0.05. The SPSS statistical package, version 24.0, was used for the analysis.
Results
A total of 154 patients were recruited to the study, of which 149 were included in the analysis. All the patients consent to participate in the study. Five patients failed to comply with the Karnofsky performance score inclusion criterion. Each center included an average of 8 patients (95% confidence interval: 5 to 11).
Sociodemographic data and medical history
Ninety-six patients (64.4%) were male, 53 were female (35.6%), and the mean age was 66 years (95% confidence interval: 64.3 to 67.9), with no difference between genders (p = 0.356). The socioeconomic level was average, low and high in 94 (71.8%), 20 (15.3%) and 17 (13%) patients, respectively.
There were no significant differences in body mass index (BMI) between males and females, the mean being 25.5 kg/m2 (95% confidence interval: 25 to 26.6), with a median of 25.1 kg/m2. BMI could not be determined in 13 patients (8.7%). Sixteen patients (10.5%) presented cachexia [16] with BMI < 20 kg/m2. Sixty-seven patients (44.1%) had BMI < 25 and ≥ 2 0 kg/m2; 41 (27%) were overweight with BMI 25-30 kg/m2; and 28 (18.4%) were obese with BMI > 30 kg/m2.
Ninety-two patients (64.3%) had good hydration; 35% had average hydration; and one patient (0.7%) showed poor hydration. The frequency of concomitant diseases was: respiratory 76 (51%), cardiovascular 55 (36.9%), gastrointestinal 17 (11.4%), genitourinary 16 (10.7%), musculoskeletal 22 (14.8%), neurologic 8 (5.4%), endocrine 30 (20.1%), hematological 7 (4.7%), dermatological 3 (2%), psychiatric 10 (6.7%), surgical 52 (34.9%) and allergy 22 (14.8%).
The cancer was located in the lungs in 106 patients (71.6%), gastrointestinal tract in 11 (7.4%), breast in nine (6.1%), prostate gland in four (2.7%), and in other body locations in 18 cases (12.2%). A total of 133 patients (89.3%) had metastatic disease at the time of assessment. The proportion of patients corresponding to each Karnofsky score was: 30, 2%; 40, 10.7%; 50, 15.4%; 60, 20.1%; 70, 27.5%; 80, 18.1%; 90, 5.4%; and 100, 0.7%.
Sixty-seven patients (47.5%) suffered associated breakthrough pain. The mean Mahler basal dyspnea score of the patients included in the study was 5.1 (95% confidence interval: 4.6 to 5.5), with a median of 5 points, a minimum of 0 and a maximum of 12. The score was 1.03 points poorer among females (95% confidence interval: 0.17 to 1.89; p =0.019). The time from initial diagnosis of basal dyspnea was 0.7 years (95% confidence interval: 0.4 to 0.9), with a median of 0.2 years. A total of 112 patients (75.2%) received treatment for basal dyspnea. The mean oxygen saturation was 92.74% (95% confidence interval: 92.04 to 93.44), with a median of 94%.
Characteristics of breakthrough dyspnea
A total of 57 patients (40.7%) presented with their first breakthrough dyspnea episode. Table 1 summarizes the characteristics of breakthrough dyspnea. There were no significant differences in dyspnea intensity between males and females (p =0.594). The events triggering dyspnea were movements (113 cases) and cough (57 cases). In 21 cases dyspnea was caused by both events.
Symptoms assessment
The intensity of basal dyspnea on the day of the inclusion visit was 3.46 points (95% confidence interval: 3.06 to 3.86), with a median of 3 points, and no differences between genders (p =0.566). Table 2 presents the mean ESAS scores observed.
 Full size
Full size 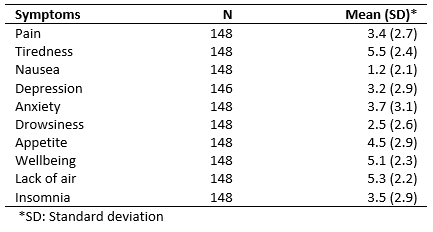 Full size
Full size Treatment for breakthrough dyspnea
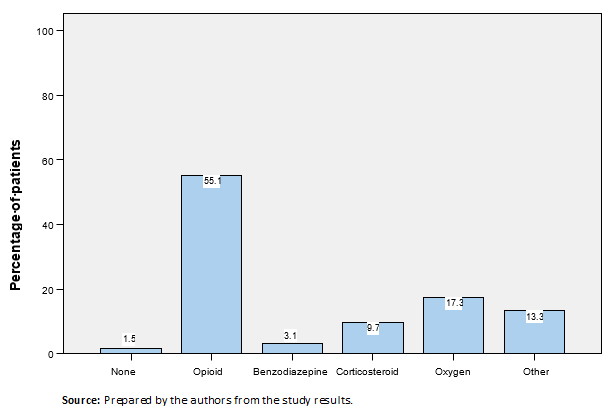
The proportion of patients who received each type of treatment for breakthrough dyspnea as first choice therapy are presented in Figure 1. Table 3 shows the drug substances and interventions prescribed as the first treatment option. Treatment was administered in monotherapy as the first choice in 119 patients (79.9%), while 19 patients (12.8%) received two drugs or interventions, six (4%) received three drugs or interventions, and five (3.4%) received more than three drugs or interventions. The drugs most frequently prescribed in monotherapy as first choice treatment were opioids (75 patients, 64.7%), followed by oxygen (22 patients, 19%), other treatments or interventions (12 patients, 10.3%), corticosteroids (four patients, 3.4%) and benzodiazepines (two patients, 1.7%). First choice treatment was modified or suspended due to lack of efficacy in 26 patients (17.5%), and due to toxicity in four patients (2.7%).
 Full size
Full size 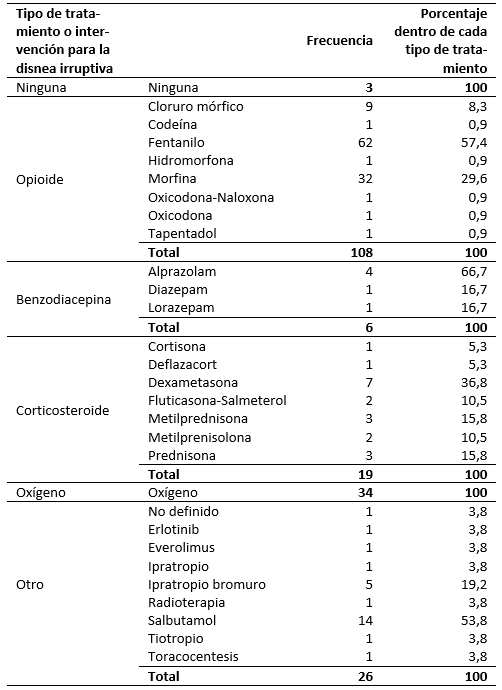 Full size
Full size Seventy-two patients (48.3%) received first choice treatment; 77 (51.7%) required second choice treatment; 37 (24.8%) required third choice treatment; 14 (9.4%) required fourth choice treatment; eight (5.4%) required fifth choice treatment; and two patients (1.3%) required a sixth treatment option. The second choice drugs were analyzed in those cases where opioids had been the first choice. In 64.9% of the cases, the second treatment option was to increase the dose of the prescribed opioid; benzodiazepines were used in 13.5%; corticosteroids in 8.1%; oxygen in 5.4%; other treatments in 5.4%; and non-pharmacological interventions in 2.7%.
Oxygen administration was seen to be associated with the presence of basal dyspnea (p =0.007), with an incidence of 21.1% versus 7.4% in the patients without basal dyspnea. The use of opioids for breakthrough dyspnea was associated with previous treatment with opioids for basal dyspnea (68.5% versus 50%; p =0.044). The use of opioids was also seen to be associated with predictable breakthrough dyspnea (70.9% versus 44.4%; p =0.01). No statistical differences were observed in the number of daily breakthrough dyspnea episodes and the type of treatment administered as first choice in monotherapy (Figure 2).
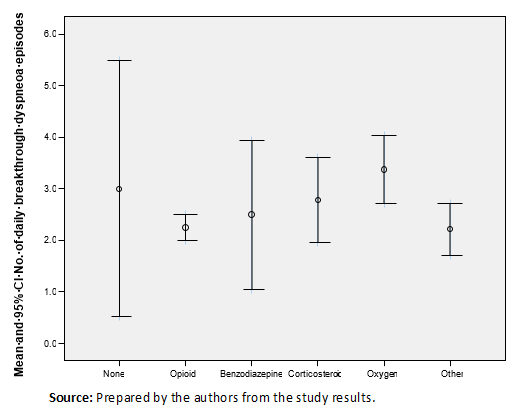 Full size
Full size Satisfaction with treatment
Eighty-seven patients (60.8%) were satisfied with the treatment for breakthrough dyspnea they were receiving. The mean satisfaction score (VAS) was 6.02 (95% confidence interval: 5.6 to 6.5), with a median of 6, and no difference between genders (p =0.285) or types of treatment (Figure 3).
Discussion
The American Thoracic Society (ATS) defines dyspnea as “a subjective experience of breathing discomfort that consists of qualitatively distinct sensations that vary in intensity. The experience derives from interactions among multiple physiological, psychological, social, and environmental factors, and may induce secondary physiological and behavioral responses” [17]. Although this is a globally accepted definition, it makes no distinction between the different forms of dyspnea, such as continuous dyspnea or episodic or breakthrough dyspnea. Different terms have been used in reference to breakthrough dyspnea: episodic, crises or attacks, acute, incident and intermittent. In patients with chronic obstructive pulmonary disease, breakthrough dyspnea is usually referred to as acute disease exacerbation [1],[2],[4],[5],[6],[8],[9],[18],[19],[20].
Despite the advances in the management of dyspnea, successful treatment is still difficult [10],[21],[22]. The etiology of breakthrough dyspnea may be related to the causes of the background dyspnea, though the disorder can also manifest in patients without background dyspnea, and with no known etiological causes [3]. Dyspnea is difficult to control independently of the underlying etiology. The control of breakthrough dyspnea is even more complex as a result of the difficulties involved in assessing the disorder, in view of its close relationship to emotional and physical factors that increase the impact on the patient [17]. Breakthrough dyspnea has a series of special characteristics that determine the choice of treatment, such as rapid onset, short duration and the repetition of episodes over the course of one day. The scientific evidence for the efficacy of the different treatments is inconclusive [11]. Opioids are recommended as first choice treatment, since they are warranted by many studies and by clinical experience. However, sufficient evidence is only available for morphine, diamorphine and dihydrocodeine; there are no comparative studies, and the starting dosage for dose titration in breakthrough dyspnea has not been established. Fentanyl could be a useful option in view of its rapid action, which is well suited to the acute way in which breakthrough dyspnea develops. However, comparative studies with other opioids are needed, since the data available to date with subcutaneous and intranasal fentanyl pectin are versus placebo [23],[24].
Benzodiazepines are recommended as a second choice, because although they are not effective in reducing the severity of breakthrough dyspnea, they are important as adjuvants for reducing anxiety or panic attack. The recommendation, therefore, is to combine them with opioids, since this option has been shown to be effective in cancer patients with dyspnea [8]. Corticosteroids are only recommended when dyspnea is caused by obstructive respiratory disease, or in dyspnea secondary to carcinomatous lymphangitis. The efficacy of oxygen therapy in non-hypoxic patients has not been demonstrated [11],[25].
In the present study, first-choice treatment consisted of opioids, followed by oxygen (17.3%). Most patients (79.9%) received monotherapy. Patients with basal dyspnea received oxygen more often than those without basal dyspnea (21.1% versus 7.4%; p =0.007), and patients with predictable dyspnea received a greater proportion of opioids than those non predictable (70.9% versus 44.4%; p =0.01). Some treatments described for breakthrough dyspnea as immunomodulators and thoracocentesis perhaps really were treatment for the basal dyspnea. The first-choice treatment for breakthrough dyspnea most widely used in Spanish clinical practice was found to be opioid monotherapy.
The fact that opioids were the treatment of choice and that breakthrough dyspnea is very limited in time (normally lasting less than 10 minutes) suggest that opioid administration routes other than the intravenous and/or subcutaneous route, with rapid onset of action, as a very attractive option for the outpatient management of breakthrough dyspnea.
The present study constitutes a global approach to the management of patients with breakthrough dyspnea in Spain. The study sample was very heterogeneous, because the patients were in different stages of malignant disease and in different treatment phases. No information was collected on the etiology of basal or episodic dyspnea, therefore, we were not able to determine whether administration and response to the treatments might also differ.
Conclusions
Despite the widespread use of opioids in clinical practice, the scientific evidence to support their use in breakthrough dyspnea is limited. Further information derived from controlled clinical trials is needed regarding the comparative efficacy of the different options in order to justify their use in clinical practice.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Notes
Author contributions
LCG: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing (original draft preparation), Writing (review and editing), Visualization, Supervision, Project administration. IDM: Methodology, Investigation, Data curation, Visualization. MRV: Investigation, Data curation, Visualization. AJJ: Conceptualization, Validation, Investigation, Resources, Data curation, Visualization, Project administration, Funding acquisition. ACA: Conceptualization, Software, Validation, Resources, Writing (original draft preparation), Writing (review and editing), Visualization, Project administration, Funding acquisition.BSL: Conceptualization, Methodology, Software, Validation, Resources, Writing (original draft preparation), Writing (review and editing), Visualization, Supervision, Project administration.
Funding
This study was sponsored by Kyowa Kirin Farmacéutica S. L. U., Madrid, Spain. The sponsor was not involved in the study design, data collection, or analysis. The decision to publish this work was the sole initiative of the authors. Also, there was no influence on the preparation, review or approval of the manuscript.
Competing interests
The authors have completed the ICMJE Conflict of Interest Declaration Form. Antonio Jiménez and Ana Cabezón declare that they belong to the Medical Department of Kyowa Kirin Farmacéutica S.L.U. The rest of the authors state that they have not received funding for the production of the article; that they have no financial relationships with organizations that might have an interest in the published article in the last three years; and that they have no other relationships or activities that might influence the published article. Forms can be requested by contacting the responsible author or the editorial management of the Journal.
Ethics
The Clinical Research Ethics Committee of the Hospital Universitario Puerta de Hierro Majadahonda has approved the protocol ARC-DIO-2015-01, in its version 3.3 of February of 2015 in its meeting of 09/A3ll5 (Minute number 308).

