Research papers
← vista completaPublished on March 24, 2021 | http://doi.org/10.5867/medwave.2021.02.8134
Efficacy of methadone for the management of postoperative pain in laparoscopic cholecystectomy: A randomized clinical trial
Eficacia de la metadona para el tratamiento del dolor postoperatorio en colecistectomía laparoscópica: ensayo clínico aleatorizado
Abstract
Background Postoperative pain management contributes to reducing postoperative morbidity and unscheduled readmission. Compared to other opioids that manage postoperative pain like morphine, few randomized trials have tested the efficacy of intraoperatively administered methadone to provide evidence for its regular use or be included in clinical guidelines.
Methods We conducted a randomized clinical trial comparing the use of intraoperative methadone to assess its impact on postoperative pain. Eighty-six patients undergoing elective laparoscopic cholecystectomy were allocated to receive either methadone (0.08 mg/kg) or morphine (0.08 mg/kg).
Results Individuals who received methadone required less rescue morphine in the Post Anesthesia Care Unit for postoperative pain than those who received morphine (p = 0.0078). The patients from the methadone group reported less pain at 5 and 15 minutes and 12 and 24 hours following Post Anesthesia Care Unit discharge, exhibiting fewer episodes of nausea. Time to eye-opening was equivalent between the two groups.
Conclusion Intraoperative use of methadone resulted in better management of postoperative pain, supporting its use as part of a multimodal pain management strategy for laparoscopic cholecystectomy under remifentanil-based anesthesia.
|
Main messages
|
Introduction
Postoperative pain management is an essential part of the anesthetic plan to address tissue injury following surgery and reduce postoperative morbidity, improving the perceived quality of care. For uncomplicated laparoscopic cholecystectomy, pain is the primary cause of prolonged hospital stay and, in the context of outpatient surgery, is the leading cause of unscheduled readmission[1],[2],[3].
Remifentanil is currently the opioid of choice for intraoperative pain management in laparoscopic surgery as it allows rapid titration resulting in predictable arousal time, independent of dose and duration of infusion[4],[5]. It does not, however, adequately cover postoperative pain and may cause tolerance and hyperalgesia[6],[7],[8],[9]. Although the whole process is not fully elucidated, activation of N-methyl-D-aspartate (NMDA) receptors is proposed as a mechanism[10],[11].
The use of NMDA receptor antagonists during surgery is purported to counter the hyperalgesia associated with the use of opioids[9],[10],[11],[12], and the use of agents such as ketamine at subanesthetic doses have resulted in decreased acute postoperative pain and less rescue opioid consumption[13],[14] included in the context of remifentanil-based anesthesia[15]. The intraoperative use of methadone is an attractive option to cover postoperative pain due to its dual effect as an agonist at opioid receptors and non-competitive antagonist at NMDA receptors[16]. This strategy may reduce the requirement for rescue morphine and its associated adverse effects[12]. Indeed, several research groups report positive and safe results with methadone in this context, including pharmacokinetic/pharmacodynamic studies in adults and children[17],[18],[19], randomized trials in major surgeries in adults and children[20],[21],[22],[23],[24],[25], and remifentanil-based laparoscopic surgery[26]. However, the risk of bias of these studies has not been evaluated formally.
A recent randomized controlled trial evaluated the performance of methadone (0.1 mg/kg) compared to morphine (0.1 mg/kg) in laparoscopic cholecystectomy and found no difference in the quality of recovery evaluated by the Quality of Recovery Questionnaire (QoR-40)[27].
Despite this, few randomized trials have tested the efficacy of intraoperatively administered methadone, compared to other opioids, when managing postoperative pain to provide evidence for its regular use or be included in clinical guidelines. Therefore, we conducted a randomized controlled trial comparing intraoperative methadone (0.08 mg/kg) to morphine (0.08 mg/kg), evaluating its impact on the total dose of rescue morphine used in post-surgical acute care and on postoperative pain.
Methods
The present study was approved by the Institutional Review Board of Hospital Naval Almirante Nef, in Viña del Mar, Chile (PO3/13), and was registered on ClinicalTrials.gov (NCT01833715).
The study was conducted at the same hospital from March to September 2013. The design is a parallel-group, randomized clinical trial. Individuals aged 18 to 70, admitted for elective laparoscopic cholecystectomy, and with American Society of Anesthesiologists (ASA) physical status score I or II were invited to participate. We excluded patients with the following characteristics: (i) kidney failure (blood creatinine > 2mg/dl), (ii) history of liver failure, (iii) body mass index (BMI) > 35 kg/m2, (iv) chronic opioid use, (v) recorded hypersensitivity to the drugs under trial and (vi) patients converted to open surgery.
Informed consent and demographical data were collected preoperatively by a member of the research team. The outcome assessors were members of the nursing staff, whom the investigators trained on applying the Numeric Rating Scale (NRS) pain scale[28],[29],[30] and on data registration. Data recorded during the procedure included the anesthetic dose administered (remifentanil and propofol), arousal time, surgical duration, hypotensive events, and ephedrine requirement. NRS was assessed and recorded at 5, 10, 15, 30, 60, and 120 minutes in the Post Anesthesia Care Unit (PACU), and 4, 8, 12, and 24 hours on the ward. We recorded the total doses of rescue medication administered (morphine in the PACU, ketorolac on the ward). The following adverse events and treatments were also recorded: nausea, vomiting, itch, urinary retention, respiratory depression, administration of antiemetics (ondansetron 4mg, IV). On discharge, patients were asked to rate their pain management quality on a four-point Likert scale (very unsatisfied to very satisfied).
Patients were randomized with an automatic computerized sequence, with an allocation ratio of 1:1, to receive either methadone or morphine. The sequence was prepared and concealed by the principal investigator. The principal investigator prepared syringes according to the allocated treatment and delivered these to the anesthesiologist in charge of the procedure. Anesthesiologists, surgeons, nursing staff, and patients were unaware of the allocated treatment.
Induction and maintenance anesthesia was based on remifentanil and propofol, titrated to attain a bi-spectral index value between 40 to 60. Following induction, all patients received dexamethasone 8 mg, ketoprofen 100 mg, and metamizole 2 g. A bolus dose of either methadone 0.08 mg/kg or morphine 0.08 mg/kg, calculated using lean body mass, was administered at the start of surgery, and these doses were chosen based on previous studies[20],[22]. We provided postoperative analgesia using a continuous infusion of ketoprofen 100 mg and metamizole 3 mg. After assessing NRS at predefined times, morphine 1 mg was administered for scores ≥ 4 in the PACU (up to 120 minutes), and ketorolac 30 mg in the ward.
Our protocol and trial registry stated our primary outcome as the total amount of rescue morphine used during the first 24 hours postoperatively; however, due to hospital policy, morphine is not administered outside of the PACU, and stay in recovery is typically two hours. We, therefore, refer to the total amount of rescue morphine and highlight that this was provided in the two hours following surgery. The secondary outcome measure was pain, determined using a Numeric Rating Scale at 5, 15, 30, 60, and 120 minutes in the PACU and at 4, 8, 12, and 24 hours postoperatively. We expected the average total dose of rescue morphine to be 4 mg in patients that had received 0.08 mg/kg of morphine during surgery. A sample size of 37 patients per group was calculated to detect at least a 25% pain reduction in the intervention group, anticipating a standard deviation (SD) of 1.5 mg in total morphine rescue dose, with a 0.05% significance level and 80% power. We randomized 86 patients anticipating 15% for possible losses (e.g., withdrawals or conversion to open surgery).
Secondary outcome variables were i) postoperative pain score in the PACU or the ward, at specified time points (resting), ii) total number of ketorolac doses in the ward per patient, iii) arousal time, iv) adverse events and treatment, v) qualification of the quality of analgesia provided by patients at discharge.
Continuous data are presented in means and standard deviations. Categorical data are presented in absolute and relative frequencies. Means ± standard deviations were compared using Student’s t-test. The number of patients (%) was compared using Fisher’s exact test, considering p ≤ 0.05 as significant. All analyses were performed using Stata 12.0 (StataCorp).
Results
One hundred and forty-six patients were assessed for eligibility, and 60 did not meet the inclusion criteria, the primary reasons being older than 70 years, a body mass index greater than 35, and a few individuals with serum creatinine over 2.0 mg/dl. A total of 86 patients were submitted to the randomization procedure, 43 allocated to the intervention group (methadone), and 43 were allocated to the control group (morphine). Three patients did not receive the intervention and instead received inhalation anesthesia (1 in the methadone group and 2 in the morphine group). Three participants in the methadone group and two in the morphine group were excluded due to a lost record, change to an open approach, or surgical complications (Figure 1).
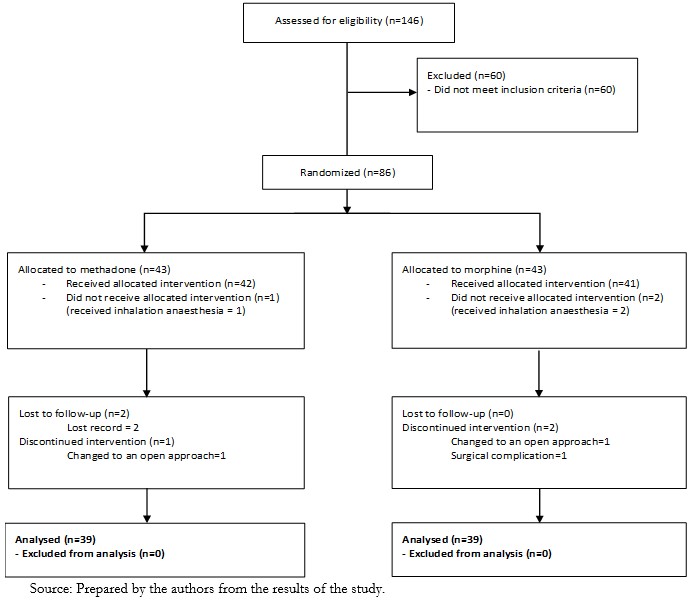 Full size
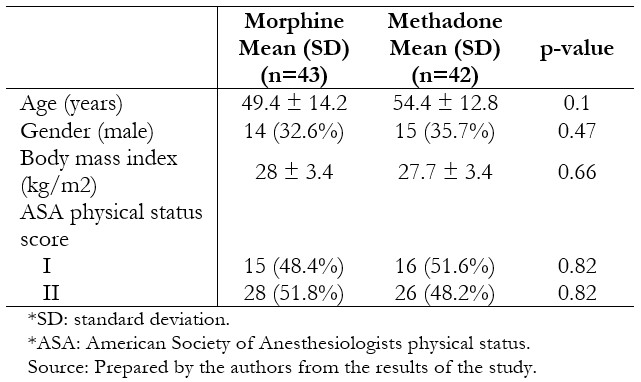
Full size Thirty-nine patients per group were included in the per protocol analysis. The groups were similar in terms of age, body mass index, gender, and physical status (Table 1). Surgical characteristics were likewise similar between the groups (Table 2).
 Full size
Full size 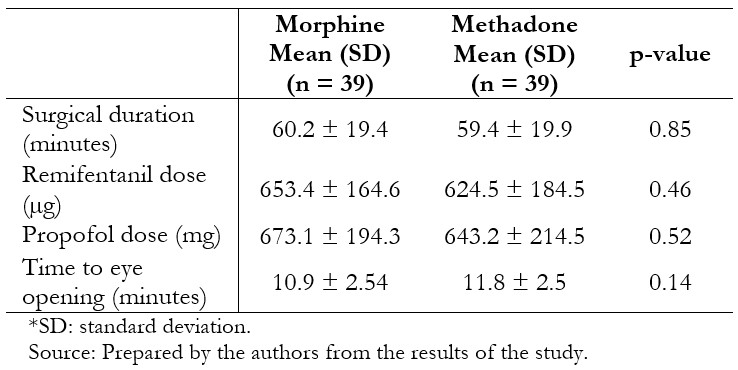 Full size
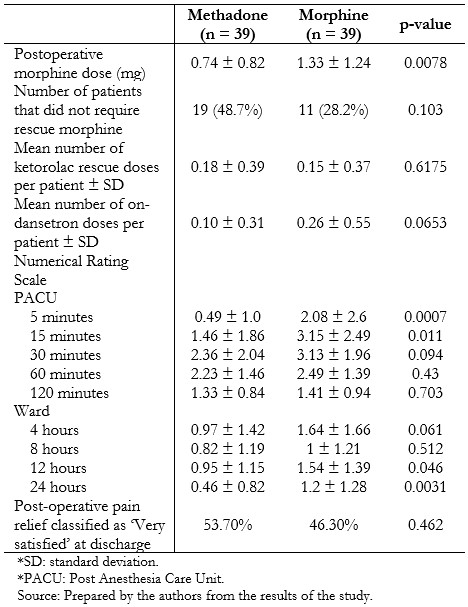
Full size Post-operative analgesia and pain scores are presented in Table 3. Individuals who had received methadone required less rescue morphine postoperatively (0.74 mg ± 0.82 mg versus 1.33 mg ± 1.24 mg, p = 0.0078), without significant differences in the amount of rescue ketorolac in the next 22 hours (0.18 ± 0.39 vs. 0.15 ± 0.37, p = 0.6175). Time to eye-opening was equivalent between the two groups. Pain scores were generally low and differed between groups at 5 and 15 minutes after recovery, and 12 and 24 hours on the ward, being lower in the methadone-treated patients in these scenarios. Fewer patients in the methadone group experienced nausea (2.6% versus 20.5%, p = 0.014), although nausea and vomiting globally were similar between the groups (Table 4).
 Full size
Full size 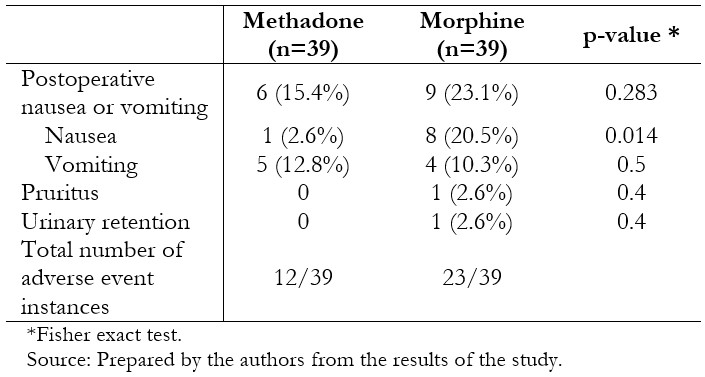 Full size
Full size Discussion
We found patients receiving 0.8 mg/kg of methadone intraoperatively required less morphine rescue for postoperative pain in the first two hours following laparoscopic cholecystectomy than those that had received 0.8 mg/kg of morphine. These patients reported less pain at 5 and 15 minutes, and 12 and 24 hours following PACU discharge, and exhibited fewer episodes of nausea. The use of methadone did not prolong the time to eye-opening.
Our results are similar to a recently published randomized trial from Moro et al., in which the same agents were compared in the context of laparoscopic cholecystectomy[27]. The primary outcome, quality of recovery at 24 hours, evaluated by the QoR-40 questionnaire, was similar between methadone and morphine treated patients. Moro et al. discussed several aspects of their finding, including high overall scores compared to previous work in more complex surgeries and multimodal approach to analgesia. We asked satisfaction with pain management at discharge and found no difference between the groups. Pain, assessed by NRS every 15 minutes while in PACU, was found not to differ between methadone and morphine treated patients; however, the authors report results as pain at rest at an unspecified time point and at 1 hour[27]. Like our work, postoperative opioid analgesia in the PACU was significantly different between methadone and morphine, though Moro and colleagues used dosage as mL (1 mg/kg in 10 mL), continuing with the treatment allocation for a given patient. While the postoperative analgesia approach was different, as all patients in our work received 1 mg of morphine per dose, the findings likely represent quite similar pain requirements as the indication for administering similar doses (maintaining NRS below 3 in Moro et al., and below 4 in our work) despite the lower doses used intraoperatively in our work (0.08 mg/kg of methadone and morphine, compared to 0.1 mg/kg). We likewise found nausea and vomiting events similar between the groups; however, we found a greater occurrence among the morphine-treated individuals when we looked explicitly at nausea. Finally, Moro and colleagues report more morphine-treated individuals with Ramsey sedation scores > 4, a relevant outcome that we did not assess.
One explanation for the difference between methadone and morphine is a less favorable pharmacokinetic/pharmacodynamic profile of morphine that leads to delayed effects relative to plasma concentrations. A greater requirement of rescue narcotics highlights the delayed onset of action of morphine in individuals who received morphine late or at the end of surgery compared to those who received morphine 40 minutes before the end of the procedure, in the context of remifentanil-based anesthesia[31]. In an investigation evaluating plasma levels and maximum effect of several opioids (pupillary diameter), methadone exhibited a more evident association than morphine, where a delay of up to two hours between peak plasma level and maximum effect was observed[16]. Another possibility is the pharmacodynamic property of methadone as both an agonist at opioid receptors and a non-competitive antagonist at NMDA receptors. NMDA receptor antagonists improve the efficacy of opioids, delaying tolerance and decreasing hyperalgesia[32]. Most of the studies comparing morphine and methadone aimed to manage chronic pain in oral and subcutaneous formulations, with proportions of 5-7:1 and 2-14:1, respectively[33],[34]. However, for the management of postoperative pain in abdominal surgeries, using a single intravenous dose, some studies have used a 1:1 ratio[20],[21],[22].
Gourlay and colleagues introduced the use of intraoperative methadone[17],[18],[20],[25],[26],[27], and many research groups have evidenced its adequate efficacy and safety in a variety of surgical contexts[19],[21],[22],[23],[24]. An IV bolus of 20 mg of methadone did not impair hemodynamic stability in patients undergoing major surgery[35]. Another advantage of methadone is that active metabolites are not produced, an issue to be considered when using morphine, especially in patients with kidney failure[36],[37],[38].
One of the limitations of the present trial is that the administration of rescue morphine was given by nursing staff rather than by patients themselves; patient-controlled analgesia likely being a more accurate representation of requirement. We appreciate that the statistically significant difference we report in terms of rescue morphine dose and reduced postoperative pain in the methadone-treated patients may represent a moderate clinical gain. This is likely due to the lower nociceptive burden in laparoscopic surgery and a multimodal approach to pain management in both groups. Methadone may represent a greater gain in surgeries associated with more intense postoperative pain[24],[25]. Future trials to evaluate the efficacy of methadone in surgeries with prolonged remifentanil exposure are desirable, and it would be advisable to incorporate standardized somatosensory tests to evaluate postoperative hyperalgesia[39],[40].
As a subjective variable, pain is most widely assessed through the NRS[41]. It is desirable that studies assessing pain, such as ours, use this scale to enable comparisons across studies facilitating future evidence synthesis efforts, such as inclusion in systematic reviews and meta-analyses.
Our results suggest advantages of intraoperative methadone compared to morphine to treat postoperative pain in laparoscopic cholecystectomy, resulting in less rescue morphine in the PACU and lower NRS scores specific periods. Further, the methadone-treated patients who exhibited less nausea are an important consideration in choosing an opioid within a multimodal treatment strategy in surgeries such as cholecystectomy.
Notes
Roles and contributions
NA: conceptualization, methodology, validation, formal analysis, research, visualization, project management, review and edition. CP: formal analysis, research, review of the original draft. MK, MM: formal analysis, research, writing original draft, review and editing. FA: conceptualization, validation, research, data collection, review and edition. JS: writing original draft, review and editing. EM: conceptualization, methodology, formal analysis, drafting preliminary project, review and edition.
Ethical consideration
The present study was approved by the Institutional Review Board of Hospital Naval Almirante Nef, in Viña del Mar, Chile (PO3/13), and was registered on ClinicalTrials.gov (NCT01833715).
Acknowledgments
We thank the Anesthesiology Department staff at Hospital Naval Almirante Nef for their collaboration with the clinical trial’s conduction.
Competing interests
The authors declare that they have no conflict of interest to disclosure.
Funding
This work was supported by the Direction of Research Universidad de Valparaíso under grant CIDI8 Interdisciplinary Centre for Health Studies.

