Research papers
← vista completaPublished on July 5, 2021 | http://doi.org/10.5867/medwave.2021.06.8231
Factors associated with mortality in patients hospitalized with COVID-19: A prospective cohort in a Peruvian national referral hospital
Factores asociados a mortalidad en pacientes hospitalizados con COVID-19: cohorte prospectiva en un hospital de referencia nacional de Perú
Abstract
Objectives To describe and assess clinical characteristics and factors associated with mortality in adult patients with COVID-19 admitted to a national referral hospital in Peru.
Methods We conducted a prospective cohort study that included hospitalized patients older than 18 years with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection diagnosis. Patients with a positive rapid serological test on admission but no respiratory symptoms nor compatible images were excluded. We collected the data from clinical records.
Results A total of 813 adults were included, 544 (66.9%) with confirmed COVID-19. The mean age was 61.2 years (standard deviation: 15.0), and 575 (70.5%) were male. The most frequent comorbidities were hypertension (34.1%) and obesity (25.9%). On admission, the most frequent symptoms were dyspnea (82.2%) and cough (53.9%). A total of 114 (14.0%) patients received mechanical ventilation, 38 (4.7%) were admitted to the intensive care unit, and 377 (46.4%) died. The requirement for ventilatory support, greater lung involvement, and inflammatory markers were associated with higher mortality. It was found that for every 10-year age increase, the risk of dying increased 32% (relative risk: 1.32; 95% confidence interval: 1.25 to 1.38). Those who were admitted to the intensive care unit and and were placed on mechanical ventilation had 1.39 (95% confidence interval: 1.13 to 1.69) and 1.97 (95% confidence interval: 1.69 to 2.29) times the risk of dying compared to those who did not, respectively.
Conclusion We found a high mortality rate among hospitalized patients associated with older age, higher inflammatory markers, and greater lung involvement.
Main messages
- Hospital mortality due to COVID-19 has been scarcely addressed in South American countries, where health conditions, economics and social systems have played a key role in this pandemic.
- This study evaluated the factors associated with mortality in patients with COVID-19 hospitalized in a national referral center in Peru.
- To date, this is the largest number of patients enrolled in Peru, partly because this research was conducted during the period of case surge in the epidemiological curve of COVID-19 patients.
- The main limitation of this study lies in the incomplete records for some variables such as adverse drug reactions, harmful habits and body mass index, which could affect the external validity of results.
Introduction
By the end of 2020, the new coronavirus disease 2019 (COVID-19) had claimed nearly 2 million deaths worldwide, according to official reports from the World Health Organization[1]. Peru is one of the most affected countries by the pandemic, with a record in April 2021 of more than 1,000 excess deaths per million inhabitants[2].
Hospital mortality associated with COVID-19 has been studied in various international cohorts[3],[4],[5],[6]. It has been determined that age, cardiovascular disease, diabetes and obesity are associated with higher mortality in patients with COVID-19[7],[8]. However, this has been scarcely addressed in South American countries, where health conditions, economics and social systems have played a significant role[9],[10],[11].
The main objective of this study was to determine the factors associated with mortality in adult patients with COVID-19 admitted to a national referral hospital in Peru. It also seeks to describe their clinical characteristics, management, and evolution, to determine differences with other country reports and to detect the groups of patients with a higher risk of mortality.
Methods
Design and population
A prospective cohort study was conducted at the Edgardo Rebagliati Martins National Hospital belonging to the Peruvian Social Health Insurance, EsSalud. This hospital is a national referral center and adapted most of its hospital capacity to COVID-19 patient care.
The study population was hospitalized patients over 18 years of age admitted through the emergency department during one month (May 22 to June 21, 2020) and a diagnosis of suspected or confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. A sample size estimation was not performed because a non-probabilistic selection was made, including the entire universe of patients in the study.
Patients with symptoms or images (radiography or tomography) suggestive of COVID-19 infection were considered suspicious cases. Patients with a positive diagnostic test by nasopharyngeal swab reverse transcriptase-polymerase chain reaction (RT-PCR), or rapid serologic test (immunoglobulin M or G) were considered as confirmed cases[12]. Patients admitted with a positive rapid serologic test with negative or absent RT-PCR and no suggestive symptomatology or compatible images were excluded and considered possible false-positive cases. The authors extracted the information from the medical records (physical or electronic) of the patients enrolled from admission to discharge or death. Periodic review of medical records facilitated the identification of participants and reduced potential losses.
Variables
Demographic (age and sex), clinical, imaging and laboratory data were recorded. After data extraction from the medical charts, the most prevalent diseases were recorded (i.e., arterial hypertension, diabetes mellitus, obesity, chronic kidney disease, ischemic heart disease, asthma, cancer, cerebral vascular disease, hypothyroidism, diffuse interstitial lung disease, liver cirrhosis, chronic obstructive pulmonary disease). In addition, obesity was considered according to two criteria:
- The body mass index according to the clinical history records of patient weight and height.
- Obesity diagnosis by the attending physician recorded in the clinical history.
The results of the RT-PCR and rapid serological tests (immunoglobulin M, immunoglobulin G or both) were included. Likewise, pre-hospitalization medication as treatment for COVID-19, symptoms and vital functions on admission were all recorded.
Additional diagnostic tests included admission laboratories (blood count, biochemical analysis, liver profile, coagulation profile, inflammatory markers, and arterial blood gases) and imaging studies. Pulmonary involvement was classified using the percentage of lung involvement and the COVID-19 Reporting and Data System (CORADS) scale[13].
Regarding hospital care variables, therapeutic management, oxygen therapy and in-hospital complications were considered. On follow-up, the need for intensive care, use of mechanical ventilation and the outcome (discharge or death) event date were recorded.
Ethics
This study represented a minimal risk for patients because no direct contact was made, and no personal information was collected that would allow them to be identified. Also, only the investigators had access to the data collected. The Research Ethics Committee approved the research protocol for COVID-19 of the Social Health Insurance. This protocol was carried out under the Helsinki declaration and the Belmont report, in which the authors agree to respect the anonymity of study patients.
Statistical analysis
The information was recorded and stored in a database using Microsoft Excel 2016 program and then exported to STATA v14 program. Frequencies and percentages were used to summarize categorical variables, while central tendency and dispersion measures were used for numeric variables, depending on data distribution.
The percentage of missing data for each variable is reported. Because the proportion of missing data for most variables was small, and because they were deemed random, we chose not to perform statistical artifacts to deal with missing data[14].
To evaluate the associations between mortality and socio-demographic, clinical and laboratory variables, hypothesis testing was performed according to the nature of independent variables. Chi-square or Fisher’s exact test was used for categorical variables, while Student’s t-test or Mann-Whitney U test was used for numerical variables.
Finally, effect size (relative risk and 95% confidence intervals) was estimated by creating Poisson regression models with robust variance[15]. We created crude models, adjusting by sex and age. In addition, socio-demographic and clinical variables were adjusted for arterial oxygen pressure/inspired oxygen fraction at admission. These variables were included in the adjusted models as they were potential confounding variables[16]. The assumption of linearity was assessed for univariate models and collinearity was assessed for the adjusted models, and no multicollinearity problems were found. A statistical significance level of 0.05 was used for all tests.
Results
Characteristics at admission
During the study period, a total of 843 adults were hospitalized with a diagnosis of COVID-19 (confirmed or suspected), of whom 30 did not meet the inclusion criteria and were withdrawn from the study. Thus, a total of 813 patients were included in the present analysis. Of these, 711 (87.5%) patients had diagnostic tests performed, and 544 (66.9%) had confirmed COVID-19. Of confirmed cases, 192 were diagnosed by RT-PCR and 352 by rapid serological test (Figure 1).
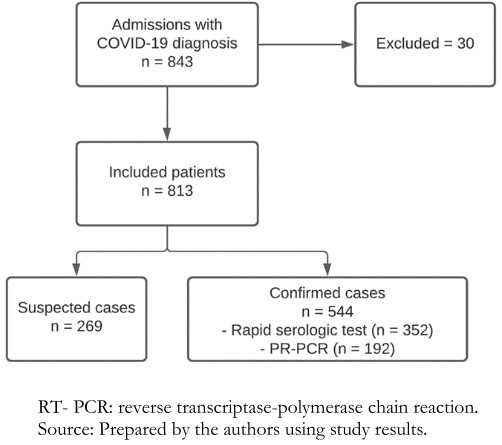 Full size
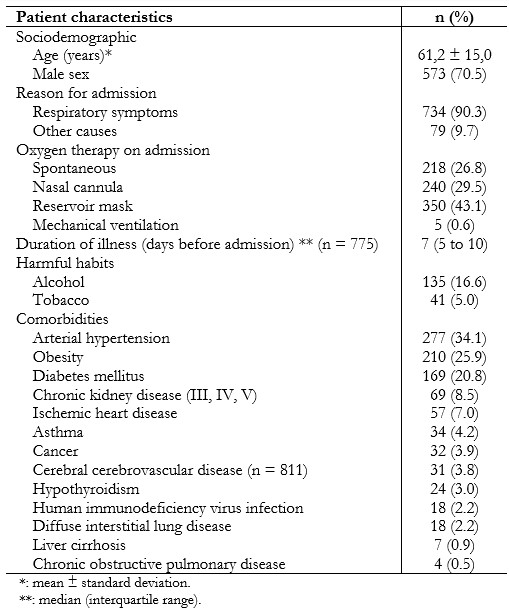
Full size The mean age was 61.2 years (standard deviation: 15.0), and 573 (70.5%) were male. The most frequent comorbidity in hospitalized patients was arterial hypertension with 34.1% (n = 277). Table 1 shows other results in each area.
 Full size
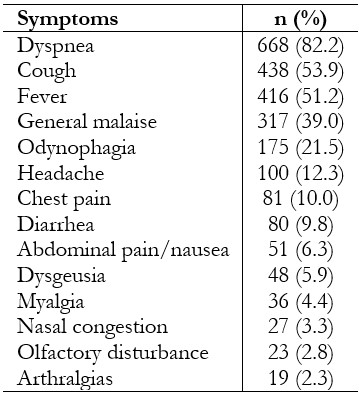
Full size Regardless of admission, most patients arrived at the emergency room with respiratory symptoms (90.3%). The median time of illness was seven days before admission (interquartile range: 5 to 10). The most frequent symptoms were dyspnea (82.2%), cough (53.9%) and fever (51.2%) (Table 2).
 Full size
Full size On admission, 374 (46.0%) patients had received previous medication as treatment for COVID-19. Thus, 286 (35.2%) took azithromycin, 148 (18.2%) ivermectin, 23 (2.8%) hydroxychloroquine, and 119 (14.6%) took combinations of these drugs, for a median of 4 days (interquartile range: 2 to 5). Regarding antiplatelets and anticoagulants, 24 (3.0%) took aspirin, 38 (4.7%) used enoxaparin, 6 (0.7%) warfarin and 26 (3.2%) received combinations of anticoagulants, for a median of 3 days (interquartile range: 2 to 5). Regarding corticosteroids, 78 (9.6%) consumed prednisone, 35 (4.3%) dexamethasone and 53 (6.5%) some combination, for a median of 4 days (interquartile range: 3 to 5).
Concerning vital functions, the median respiratory rate was 24 breaths per minute (interquartile range: 22 to 28), heart rate was 92 beats per minute (interquartile range: 82 to 104), and systolic blood pressure was 100 millimeters of mercury (interquartile range: 100 to 120). Most patients required a reservoir mask (43.1%) or nasal cannula (29.5%) at admission. The median oxygen saturation on admission was 89% (interquartile range: 85 to 92%).
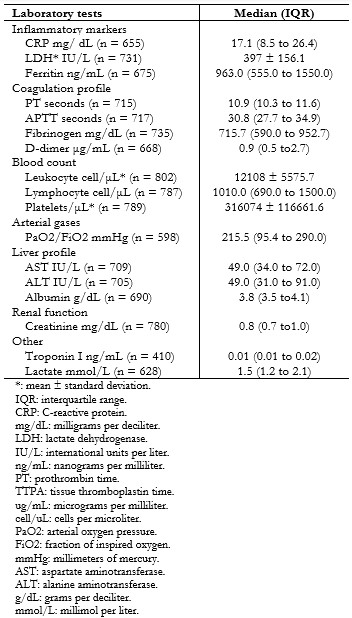
All hospitalized patients underwent some imaging test, and computed tomography was performed in 93.7% of patients. Pulmonary involvement was assessed using the CORADS classification, and it was noted that: 10 (2%) had non-suggestive images (CORADS scale 1 and 2), 17 (2.1%) indeterminate (CORADS scale 3) and 779 (96.0%) a suggestive pattern (CORADS scale 4, 5 and 6). The mean percentage of compromised lung parenchyma on computed tomography was 55.4% (standard deviation: 18.7). Table 3 shows laboratory tests performed on admission.
 Full size
Full size Management and evolution during hospitalization
During hospitalization 462 (56.8%) patients received azithromycin, 97 (11.9%) hydroxychloroquine, 168 (20.7%) ivermectin, 498 (61.3%) received prophylactic enoxaparin, 172 (21, 2%) therapeutic enoxaparin, 18 (2.2%) unfractionated heparin, 200 (24.6%) received dexamethasone, 243 (29.9%) methylprednisolone in pulse doses (125 to 500 milligrams) and 233 (28.7%) in lower doses. The median days of corticosteroid treatment was 3 (interquartile range: 2 to 5).
An electrocardiogram was performed in 143 (17.6%) of the patients of which, 116 had normal findings or one that correlated with their history, and 27 had a new finding. From the latter, 18 patients had a QT interval prolongation. No association was found between in-hospital administration of azithromycin and hydroxychloroquine and QT interval prolongation (p-value = 0.928 Chi-square and p-value = 0.121 Fisher’s exact test, respectively).
Most patients (782 equivalent to 96.2%) received antibiotics during hospitalization. Antibiotic treatment included: 486 (59.8%) of the patients receiving ceftriaxone, 118 (14.5%) piperacillin-tazobactam, 64 (7.9%) meropenem, 64 (7.9%) vancomycin and 114 (14.0%) receiving another antibiotic scheme.
During hospitalization, 215 (26.5%) patients had hospital-acquired pneumonia, 151 (18.6%) had acute kidney injury, 79 (9.7%) developed delirium, 17 (2.1%) had a venous thrombotic process, and 20 patients had other complications. The median time of illness in which patients had an increased oxygen requirement was at day 10 (interquartile range: 7 to 14).
Concerning the outcomes of interest, 114 (14.0%) of hospitalized patients received mechanical ventilation, 38 (4.7%) were admitted to the intensive care unit, and 377 (46.4%) died. The median length of intensive care unit stay was six days (interquartile range: 4 to 11), and the median length of hospital stay was ten days (interquartile range: 6 to 17).
Factors associated with COVID-19 mortality
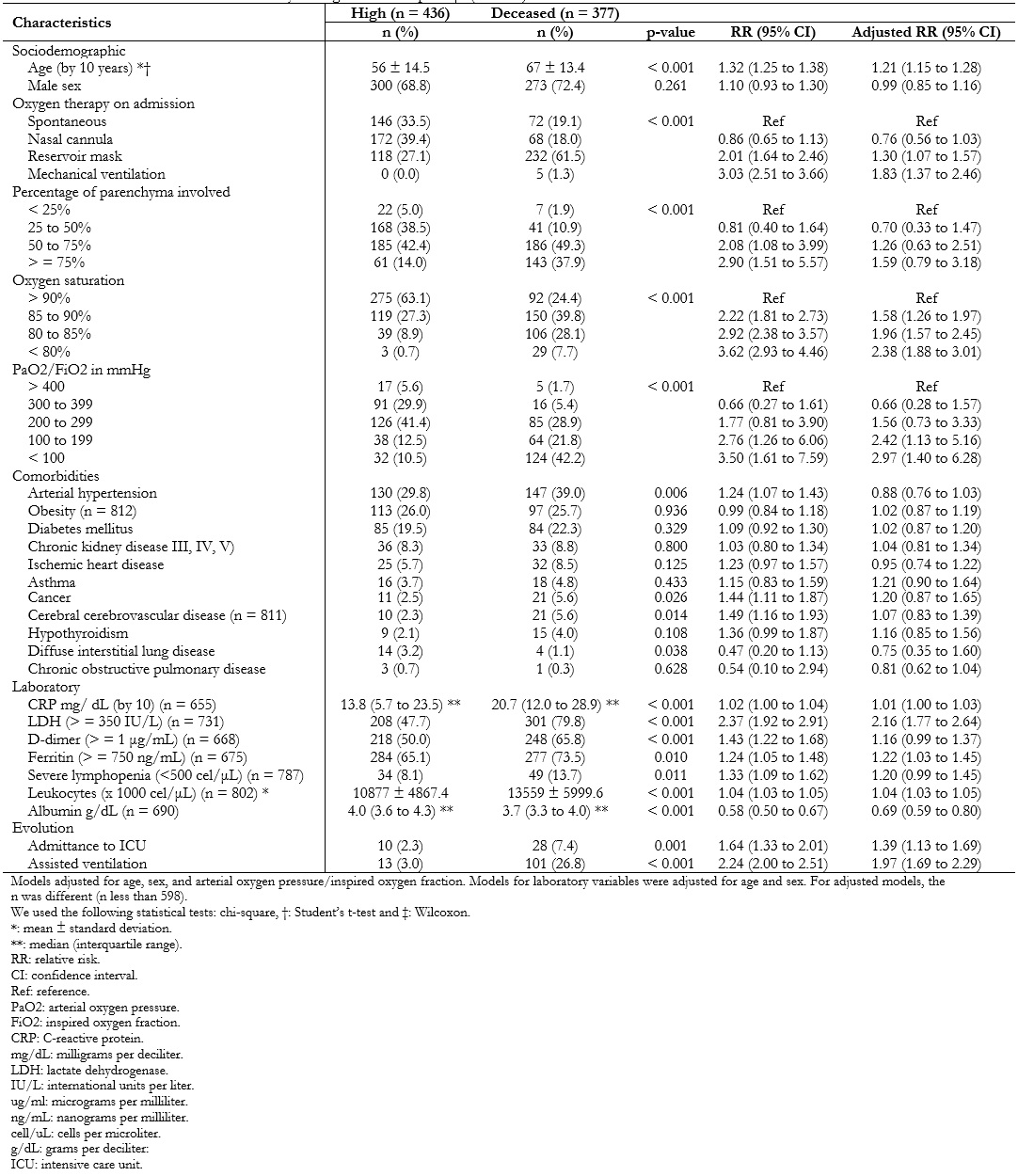
In a bivariate analysis, it was found that there was a higher proportion of male sex among deceased patients and a higher age mean. Concerning admission characteristics, requiring ventilatory support and greater pulmonary involvement in the computed tomography scan were associated with greater mortality. However, there was no association between time of illness at admission and mortality (p-value = 0.9087 Wilcoxon).
Likewise, certain comorbidities – such as arterial hypertension, cancer, ischemic heart disease, cerebral vascular disease and hypothyroidism – were associated with higher mortality. This association was lost for all comorbidities after adjusting for age, sex, and arterial oxygen pressure/inspired oxygen fraction.
Several inflammatory markers such as C-reactive protein, lactate dehydrogenase, D-dimer, ferritin, lactate, among others, were directly associated with higher mortality (Table 4).
While assessing these associations using Poisson regression models, it was found that for every ten years that age increased, the risk of death increased by 32% (relative risk: 1.32; 95% confidence interval: 1.25 to 1.38). This association was maintained when adjusted for sex and arterial oxygen pressure/inspired oxygen fraction at admission (relative risk: 1.21; 95% confidence interval: 1.15 to 1.28).
In addition, those who required intensive care unit admission and mechanical ventilation had 1.39 (95% confidence interval: 1.13 to 1.69) and 1.97 (95% confidence interval: 1.69 to 2.29) times the risk of dying compared to those who did not receive such support. This effect was maintained after adjusting for age, sex and arterial oxygen pressure/inspired oxygen fraction on admission (Table 4).
 Full size
Full size Discussion
This prospective cohort aimed to establish factors associated with mortality in hospitalized adult patients with COVID-19 diagnosis in a hospital in Peru. It also sought to describe their clinical characteristics, management, and evolution. To date, this is the largest COVID-19 cohort published at a national level[17],[18]. Among other factors, this was achieved because of hospital capacity and because it was conducted during a period of increased cases in the epidemiological curve of patients diagnosed with COVID-19, as reported in the situation room of the Ministry of Health[19].
Our study reports demographic characteristics like those already evidenced in other cohorts carried out worldwide and in Peru[3],[17],[18], where the male sex presents a higher percentage of hospitalized patients compared to the female sex, and that the meantime of illness at hospital admission is seven days.
Regarding comorbidities, arterial hypertension is the most frequent, followed by diabetes and obesity. However, the percentage of obesity described is low (25.9%) compared to other studies (over 40%), and no association with mortality was found[17],[20]. One explanation for this could be an underreporting of body mass index measurements in medical records. In addition, the lack of personnel and high demand for care during the pandemic made it difficult to take the usual anthropometric measurements in hospitalized patients. This could explain why, unlike other studies, we did not find an association between obesity and mortality.
The symptoms most frequently described on admission were dyspnea, cough and fever; in contrast to hospitalized patients from China[21] and the United Kingdom[5], where fever was identified as the most prevalent symptom, followed by cough and dyspnea. As the most frequent symptom on admission, dyspnea reflects that patients came to the hospital later than in other countries.
Chest computed tomography was a rapid diagnostic support method due to the lack of diagnostic tests available in our hospital[22]. Regarding laboratory results, leukocytosis was evidenced in 62.12% of patients, of which 76.47% had relative lymphopenia with values above a study in China (40%)[4]. On the other hand, a cohort in the United States described a mean C-reactive protein of 8.35 milligrams per deciliter, associated with the rest of inflammatory markers, lower than in our population (17.1 milligrams per deciliter)[23]. These findings show that most of our patients present with a greater systemic response.
During this study, hydroxychloroquine was less prescribed (11.9%) compared to other national studies. In addition, there was greater use of ivermectin (20.7%), which may be because, since the beginning of the pandemic, the therapeutic scheme has been modified in the Ministry of Health management guidelines[24].
Regarding corticotherapy, more than half of our patients received methylprednisolone without a schedule of dosage and days of administration. Dexamethasone was used in a quarter of our patients. The prescription of this corticosteroid is explained through a preliminary report of a study, where a benefit was observed in the reduction of 28-day mortality in hospitalized patients requiring oxygen or mechanical ventilation[25]. Corticosteroid therapy in these patients is justified by the systemic inflammatory response observed in patients with COVID-19. However, current data mention that circulating cytokines are much lower than observed in other entities such as septic shock, multiple trauma or cardiac arrest[26].
The use of anticoagulation in this disease has been justified by multiple reports of thromboembolic events in hospitalized patients with COVID-19 and due to its potential benefit in critically ill patients. However, high numbers of major bleeding events are also reported, so the indication should be individualized[27]. More than half of our patients received some anticoagulation regimen, and no major bleeding events associated with anticoagulation were reported.
Regarding adverse drug reactions, the safety profile of drugs used in our cohort (such as hydroxychloroquine alone or in combination with azithromycin) involves the cardiologic risk of QT interval prolongation[28] that increases with concomitant use of antibiotics, antiarrhythmics, anesthetics, muscle relaxants, among others. In addition, other adverse effects such as hepatic toxicity, abdominal pain, diarrhea, nausea and/or vomiting have been reported[29]. Adverse reactions evaluation in the context of new diseases such as COVID-19 requires intensive pharmacovigilance, which was not performed in this study. However, this is being now performed by the Institutional Reference Center for Pharmacovigilance and Technovigilance of the Social Health Insurance[30].
Regarding in-hospital complications, it was observed that the most frequent complication was bacterial pneumonia (26.5%). This finding contrasts with the percentage of patients who received an antibiotic regimen for suspected in-hospital infection, where most failed to identify a pathogen (36.4%). A systematic review reported a low prevalence of bacterial co-infection in hospitalized patients with COVID-19, being identified in 3.5% at admission and 15.5% during hospitalization. This study concluded that empirical antibiotic treatment is not justified in most hospitalized patients with COVID-19[31]. This is an aspect that should be kept in mind since bacterial resistance could be a major problem in the near future.
Our study found that only 4.7% of our patients were admitted to the intensive care unit, and 14% received ventilatory support. This could be explained by the availability of mechanical ventilators in hospital areas outside the intensive care unit within the study hospital. Even so, only 14% of our patients received mechanical ventilation support. This report is similar to other national studies (10.18% and 16.1%)[17],[18] and lower compared to international studies conducted in high-income countries[3],[4],[5],[6]. Again, this could be due to our health system collapse, where not all patients who required admission to the intensive care unit were able to access ventilatory support.
This study found high mortality (46.4%) that is much higher than reported in New York (39%), Wuhan (28%) and London (29%)[3],[4],[5]. This phenomenon may be attributed to critical admission status, lack of access to ventilators, insufficient intensive care unit beds and other potential factors identified in previous studies[17],[18],[32].
Among mortality-associated factors, it is described a higher proportion of male deaths (72.4%), consistent with a systematic review that showed significant mortality risk for males compared to females (relative risk: 1.86)[16]. A cohort study in China reported a lower risk of death in the female sex (odds ratio: 0.44; 95% confidence interval: 0.34 to 0.58) after adjusting for age and comorbidities[33]. However, when the adjusted analysis was performed, no association was found. One possible explanation is that the sex ratio is dissimilar between age groups, as suggested by Bhopal SS and Bhopal R[34].
Age was associated with higher mortality. In our series, the highest proportion of deaths corresponded to patients older than 60 years. For every ten years increase in age, the risk of death increased by 32%. This was also found in a large series of Chinese population data in which the age groups 60 to 69, 70 to 79 and over 80 years had 3.6%, 8% and 14.8% mortality, respectively[35].
The present study found an association between mortality and different proinflammatory markers (C-reactive protein, ferritin, lactate dehydrogenase, leukocytosis), which may serve as early biomarkers of severity in COVID-19[36]. In addition, we found an association between mortality and hypoxemia on admission and with the requirement for oxygen therapy, especially in those patients who required a reservoir mask or mechanical ventilation. This is evidenced in a retrospective cohort in which 68% of patients with peripheral oxygen saturation of 90% or less did not survive after oxygen supplementation. Meanwhile, 98% of those patients with partial oxygen saturation greater than 90% survived (log-rank P < 0.001)[37].
Partial oxygen saturation on admission was also a predictor of mortality. In our series, those patients admitted with partial oxygen saturation less than 80% had higher mortality than those admitted with greater than 90%. Other studies do not describe such low partial oxygen saturations on admission, which could be explained by seeking treatment delay to the emergency department and the high service delays due to service collapse[38].
It is important to carry out additional studies in our region that concentrate a greater number of patients with consistent results of the factors associated with COVID-19 mortality. With these results, it is possible to reorganize the hospital care system to manage patients with higher mortality risk factors differentially. These measures are fundamental in Latin American regions where there is still low coverage of vaccination programs due to the scarcity of vaccines.
Our study has several strengths. First, it was carried out in the national referral hospital for COVID-19 patient care, which has the largest hospital capacity in the Peruvian social security system. Second, a systematic record was kept of patient data from their admission until their final outcome, using the electronic medical history system. This system contains a record of clinical data, laboratory tests and imaging performed. However, it has certain limitations since data was obtained through the electronic system, there was an incomplete record of some variables (adverse drug reactions, harmful habits and body mass index) that could affect the external validity of the results obtained.
Conclusion
We report the largest series of patients hospitalized with COVID-19 in Peru. In addition, the population evaluated presented high mortality (46.4%), which differs from what is previously reported in developed countries.
Mortality was associated with age (over 60 years), inflammatory markers and respiratory involvement.
Finally, a reorganization of the hospital care system is necessary to assess differentiated management to patients with higher mortality risk factors.

