Research papers
← vista completaPublished on August 30, 2021 | http://doi.org/10.5867/medwave.2021.07.8454
Clinical and epidemiological characteristics of mothers with COVID-19 and their neonates: vertical transmission
Características clínicas y epidemiológicas de madres con COVID-19 y sus neonatos: transmisión vertical
Abstract
Introduction COVID-19 disease can affect women at any stage of pregnancy, and newborns could become infected with SARS-CoV-2 through vertical or horizontal transmission.
Objective To determine clinical and epidemiological characteristics of mothers with COVID-19, associated neonatal outcomes, and to evaluate SARS-CoV-2 vertical transmission.
Methods We conducted an observational, descriptive, cross-sectional study. We included all mothers with positive serology for SARS-CoV-2 and their newborns at the Hospital Regional Docente de Trujillo from April 18 to September 30, 2020. Variables were collected from the medical records, and descriptive statistics were used for the analysis.
Results A total of 647 mothers and 656 neonates were enrolled. Of all live births, 85.3% and 14.7% were term and preterm neonates, respectively. We found 1.7% (11/656) of newborns with positive RT-PCR for SARS-CoV-2; and that 27.3% (3/11) of these neonates required hospitalization. Neonatal mortality was 4/656 (0.6%), and no case was attributed to COVID-19. Of all mothers affected with COVID-19, 95.7% were asymptomatic, and 4.3% presented clinical symptoms attributed to COVID-19, most of which were mild. The most frequent obstetric complications were preeclampsia-eclampsia, prelabour rupture of membranes, and acute fetal distress. All the mothers were discharged.
Conclusion We found 1.7% of newborns with positive RT-PCR test for SARS-CoV-2; and that 20.1% of these neonates were hospitalized. The most frequent morbidity was neonatal sepsis and prematurity. The infection was mild among newborns, showing a 0.6% overall mortality, with no cases attributed to COVID-19. We found that only 5% of mothers presented symptoms, most of which were mild to moderate symptoms. There was no record of maternal mortality in this study group. It is not possible to conclude whether vertical transmission or intrapartum-acquired infection is responsible for neonatal COVID-19 infections.
Main messages
- Little is known about SARS-CoV-2 infection in neonates born to mothers with COVID-19. This work describes the main clinical and epidemiological characteristics, providing a basis for future interventions in children born to mothers with COVID-19.
- The study's limitations were the lack of molecular tests to diagnose SARS-CoV-2 infection in pregnant mothers. In addition, there may be a bias in data collection because of the retrospective design.
- The percentage of neonates with a positive RT-PCR test for SARS-CoV-2 was low, and there was no mortality attributed to COVID-19.
Introduction
Coronavirus 19 disease (COVID-19) is caused by coronavirus-2, severe acute respiratory syndrome (SARS-CoV-2). It was first reported to the World Health Organization (WHO) in Wuhan, China, on December 31, 2019, as pneumonia of unknown cause. The coronavirus consists of a single strand of ribonucleic acid and a glycoprotein envelope. It has 79% nucleotides identical to SARS-CoV and 50% with the coronavirus that causes the Middle East respiratory syndrome (MERS-CoV) [1].
Almost one year after the first case was diagnosed in the Region of the Americas, the Pan American Health Organization (PAHO) and WHO reported 41 141 507 confirmed cases and 947 455 deaths. As of January 21, 2021, 1 068 802 cases and 38 931 deaths were reported in Peru. In La Libertad, there are 38 662 confirmed cases and 2540 deaths [2]. On January 30, 2020, WHO declared COVID-19 an international public health emergency, and on March 11 of the same year, it was declared a pandemic. In Peru, a national emergency was declared on March 15, 2020, after the first case was diagnosed in the national territory on March 6.
According to current evidence, COVID-19 is transmitted rapidly by close contact through respiratory droplets, fomites, and aerosols. For these reasons, it can affect pregnant women at any stage of pregnancy. Gestation presents a unique immunological situation characterized by increased susceptibility to some infections, including respiratory pathogens. Therefore, pregnant women may be at increased risk for severe COVID-19 compared to the general population. However, the available information on current COVID-19 is scarce, both in pregnant women and in neonates. It can be suggested that pregnant and non-pregnant women affected by SARS-CoV-2 may have a similar clinical course. Evidence on the possibility of vertical transmission is still unclear, especially when the pregnant woman is infected during the third trimester and the risk of horizontal transmission in the neonate is the same as in the general population [3][4].
The possibility of transmission of SARS-CoV-2 from mother to child is a concern, as there is a theoretical risk of vertical transmission of SARS-CoV-2 similar to SARS-CoV infection through angiotensin-converting enzyme II (ACE2) receptors that are widely distributed in the placenta. There is no evidence of vertical transmission in 46 reported cases, supported by negative tests in samples taken from amniotic fluid, cord blood, pharyngeal swab of neonates, and breast milk samples. It is important to mention that in all cases, pregnant women acquired COVID-19 in the third trimester of gestation [5].
Due to the scarcity of reagents to detect the virus, there are places where the vast majority of patients only undergo a pharyngeal swab for reverse transcriptase-polymerase chain reaction (RT-PCR), which is not sufficient to prove or rule out the presence of vertical transmission [6].
Given the possibility of vertical transmission, we set ourselves the following research problem: What are the clinical and epidemiological characteristics of mothers with COVID-19, their neonates, and the potential risk of vertical transmission of SARS-CoV-2?
The Regional Teaching Hospital of Trujillo, located in La Libertad, Peru, is a category III-1 hospital. This facility has been designated to care for the population affected by COVID-19, including infected pregnant women and their newborns. Since this is a novel disease, we know very little about newborns infection. We are concerned about the clinical manifestations and evolution in this stage of life, considering this is a particularly vulnerable population due to the immaturity of their immune system – which is a particular concern in premature infants.
There is still very little information on whether SARS-CoV-2 has transplacental or vertical transmission. Few isolated case reports have been published; however, it is reasonable to think that any neonate born to a SARS-CoV-2-positive mother can become infected in utero or through perinatal transmission, so all necessary protective measures should be taken [1]. It should be considered that neonates are at very high risk of SARS-CoV-2 infection due to their immature immune system, and vertical transmission cannot be ruled out [7]. That is why it is necessary to study the greatest possible number of cases, given that the available information changes every day and the risks in newborns could be underestimated.
The overall aim of the present study is to determine the clinical and epidemiological characteristics of mothers with COVID-19, their neonates, and the vertical transmission of SARS-CoV-2. The specific objectives are:
a) To identify the clinical and epidemiological characteristics of neonates born to mothers with COVID-19.
b) To identify the clinical and laboratory characteristics of mothers with COVID-19.
c) To assess the vertical transmission of SARS-CoV-2.
Several authors conducted small studies (between 1 and 18 patients) on neonates born to COVID-19 positive mothers during the third trimester of gestation. In these studies, the authors took samples of amniotic fluid, placenta, umbilical cord blood, and pharyngeal swab – all of which were negative [6][8][9][10][11][12][13].
In their analysis, Cheruiyot et al. [3] included five studies (16 pregnant women with COVID-19) conducted in China. All cases were pregnant women with onset of symptoms in the third trimester, but none of the samples taken from the neonates were positive for SARS-CoV-2. Similarly, Di Mascio et al. [14] conducted a systematic review in Italy, including six studies of pregnant women with COVID-19 (41 pregnant women). None of the newborns showed signs of vertical transmission during the follow-up period. Similarly, in China, Yan et al. [15] conducted a multicenter study involving 116 pregnant women with confirmed COVID-19. Eighty-six percent of the neonates had pharyngeal swabs, amniotic fluid, and cord blood samples taken to seek for SARS-CoV-2. All results were negative.
Yu et al. [16] in China report a case of a neonate with a positive RT-PCR result for SARS-CoV-2 in a pharyngeal swab sample taken 36 hours after birth. The patient presented mild respiratory distress and chest radiography that showed mild pulmonary involvement. The patient had positive RT-PCR in a nasopharyngeal swab. However, the placenta and umbilical cord blood tests were negative, so it is doubtful that this was a vertical infection.
Dong et al. [17] in China reported a case of a newborn with elevated IgG and IgM antibodies for SARS-CoV-2 from a mother positive for COVID-19. Similarly, Zeng et al. [18] reported two neonates with elevated IgG and IgM in blood samples at birth. Serum tests have a high sensitivity (88.2%) and specificity (99%) for IgM and even higher sensitivity (97.8%) and specificity (97.9%) for IgG. IgM is not transferred placentally, and elevated IgM in the newborn suggests intrauterine infection. IgM is elevated three to seven days after infection and, and if the sample is taken at birth, a positive test supports intrauterine infection.
We are going through a pandemic that is causing many deaths worldwide, produced by a new coronavirus. SARS-CoV-2 has been associated with severe lower respiratory tract infection, acute respiratory distress syndrome, and death [19].
Clinical manifestations of SARS-CoV-2 infection include fever, dry cough, dyspnea, chest pain, fatigue, and myalgia, in addition to sensory manifestations such as anosmia and dysgeusia, which are common. Less common symptoms include headache, dizziness, abdominal pain, diarrhea, nausea, and vomiting. Skin rash, generalized urticaria, and vesicles resembling chickenpox also have been described. In severe cases, hematological alterations, lymphopenia and neutrophilia, moderate thrombocytopenia, prolonged prothrombin time, disseminated intravascular coagulation, and thromboembolic events have been reported.
Approximately 75% of patients may present with bilateral pneumonia. Chest computed tomography may show ground-glass opacities, consolidation, and interstitial abnormalities producing a severe acute respiratory syndrome that may lead to mechanical ventilation and death. Severe complications such as hypoxemia, acute respiratory distress syndrome, arrhythmias, shock, acute cardiac damage with fulminant heart failure, stroke, and acute renal damage have been reported in patients with COVID-19. Children are usually asymptomatic, and one of the most frequent symptoms is vomiting [19][20].
Neonates may be asymptomatic or present symptoms such as fever, rhinorrhea, cough, tachypnea, diarrhea, and feeding intolerance, generally with ad integrum recovery. However, the evolution of the disease in some cases may be severe and require mechanical ventilation [21][22].
Current evidence shows that COVID-19 during early pregnancy is no more severe than among non-pregnant women. Similar patterns of symptomatology, disease severity, and outcomes occur. However, some infected pregnant patients may develop severe diseases requiring mechanical ventilation and even lead to death [19].
The US National Institute of Health [23] refers that clinical symptoms may vary according to disease severity and divides it into the following categories:
a) Mild COVID-19: defined as the absence of viral pneumonia and hypoxemia. They may have fever, cough, sore throat, headache, general malaise, muscle pain, nausea, vomiting, diarrhea, and loss of taste and smell.
b) Moderate COVID-19: for those patients with viral pneumonia but without hypoxemia.
c) Severe COVID-19: when patients present dyspnea, hypoxemia, or pulmonary infiltrates greater than 50%.
Rapid and accurate detection of COVID-19 is crucial to control community and hospitals outbreaks. Current diagnostic tests include RT-PCR, real-time RT-PCR (rRT-PCR), and reverse transcription loop-mediated isothermal amplification (RT-LAMP) [24].
Vertical transmission refers to the passage of a pathogen from mother to baby before or after delivery. It mainly includes transmission by germ cells or blood through the placenta during pregnancy and postpartum through breastfeeding [10].
There is controversy about whether SARS-CoV-2 infection can be transmitted from mother to fetus because most reports are from pregnant women during the third trimester. Yu et al. [25] report two cases of negative SARS-CoV-2 in amniotic fluid in women infected during the first trimester (eight weeks). Upon reaching the second trimester, they underwent amniocentesis. PCR, IgM, and IgG were performed on the sample, and all tests were negative. Although SARS-CoV-2 was not detected in the amniotic fluid of these patients, the possibility of vertical transmission during the first and second trimester of gestation cannot be ruled out for several reasons:
- RNA is less stable in amniotic fluid than DNA.
- The number of patients was too few to reach a definitive conclusion.
- Transient positive results in amniotic fluid for Zika virus and other RNA viruses have been reported.
- The virus may have been undetectable in amniotic fluid due to insufficient gestational age.
To date, there is no evidence of the consequences of SARS-CoV-2 infection in pregnant women, but the outcomes could be severe in the mother and infant. Some authors recommend that newborns born to COVD-19 positive mothers should be isolated for at least 14 days [26][27]. In contrast, Elwood et al. [28] (on behalf of the infectious diseases committee of the Society of Obstetricians and Gynaecologists of Canada) recommend that management should be decided according to the best evidence to date and the conditions of the mother and infant. Universal isolation of the infant of a confirmed or suspected mother is not recommended. This would depend on the availability of resources and the family decision, who may choose to separate the infant from the mother until she completes her isolation. The mother may also wear a mask, perform proper handwashing to care for her infant and choose to breastfeed directly. The mother may transfer antibodies to her infant through breastfeeding. However, there is no evidence on this statement, and the potential benefit is still unclear.
The available data on COVID-19 in pregnant patients do not provide a clear conclusion on mother and child clinical implications. The reports presented so far are favorable, but the fetal and maternal risks may not be fully considered. So far, experience is limited to mothers who developed the disease in late gestation, and the fetal consequences of long-standing infections occurring in early gestation are unknown [29].
Methods
This is an observational, descriptive, cross-sectional study conducted at the Hospital Regional Docente de Trujillo, Peru. This is a category III-1 facility designated to care for patients with COVID-19 in the region.
The included population consisted of all mothers with positive serology for SARS-CoV-2 and their respective newborns at the facility between April 18 and September 30, 2020.
All neonates born to mothers with COVID-19 who delivered at the facility were retrospectively identified. The study included all those who had the necessary medical records and the neonate's RT-PCR result. Patients with incomplete neonatal or maternal medical records were excluded from the study.
The study was of the census type, so no sample size calculation was performed. We worked with all mothers and their newborns that met the inclusion criteria.
The information on the neonatal variables was taken from the medical records: gestational age by physical examination, birth weight, Apgar score at one and five minutes, whether hospitalization was required, morbidities, and mortality. Maternal variables such as condition at discharge and obstetric complications, the results of the anti-SARS-CoV-2 antibody test, and the clinical picture by COVID-19 were also recorded and stratified considering:
- Mild: the absence of pneumonia and hypoxemia and the presence of symptoms such as fever, cough, sore throat, general malaise.
- Moderate: pneumonia without hypoxemia or with the requirement of non-invasive oxygen therapy.
- Severe: respiratory failure with hypoxemia, requiring mechanical ventilation and admission to the intensive care unit.
The data of the mothers and their newborns were taken from the clinical records. They were documented through a coded research form prepared for this purpose to protect the patient's anonymity. The data were collected simultaneously by two investigators and subsequently reviewed by two others to ensure the quality of the information and avoid measurement bias.
Evidence of vertical transmission was determined by rRT-PCR test on nasopharyngeal swabs from the newborns, taken within two hours of birth. The samples were sent to the referral laboratory of La Libertad.
We followed The Health Directive of the Ministry of Health [30] recommendation. All pregnant women attending a health facility should undergo a rapid test to confirm or rule out a COVID-19. In compliance with this, if the pregnant woman had positive anti-SARS-CoV-2 antibodies, she was admitted to the study hospital and, if negative, she was referred to other health facilities. Likewise, all pregnant women referred from other centers with a positive rapid serological test were admitted. The LUNGENE COVID-19 IgG/IgM rapid test cassette produced in China by Hangzhou Clongene Biotech was used to detect anti-SARS-CoV-2 antibodies. The latter is a lateral flow immunochromatography test for the qualitative detection of IgG and IgM antibodies to SARS-CoV-2 in whole blood, serum, or plasma.
The results were presented in contingency tables. For statistical analysis, continuous variables were expressed as median and interquartile ranges and simple ranges. Categorical variables were presented in number and percentage using the SPSS statistical program for Windows version 23.
The present study was approved by the Chief of the Teaching and Research Support Office of the Hospital Regional Docente de Trujillo, who considered that the project met the necessary technical and ethical criteria and therefore authorized its execution. Patient information was collected using coding to identify the participants and respect all the information's confidentiality and veracity.
Results
We included 656 neonates born to 647 mothers with COVID-19 infection. The disparity is due to nine twin births.
Table 1 shows the characteristics of 656 neonates. It shows that the median and interquartile range (IQR) of gestational age (by physical examination) in weeks was 39 (38 to 40); 85.4% were term neonates, and 14.6% were preterms. The median and interquartile range of births weight in grams was 3190 ± 780 (2770 to 3550), with 10.6% of low birth weights (birth weight less than 2500 grams). We found that 3.1% and 0.5% of newborns had 1-minute and 5-minutes Apgar scores of less than seven, respectively. In addition, 20.1% of neonates were hospitalized, and 1.7% had a positive nasopharyngeal RT-PCR test.
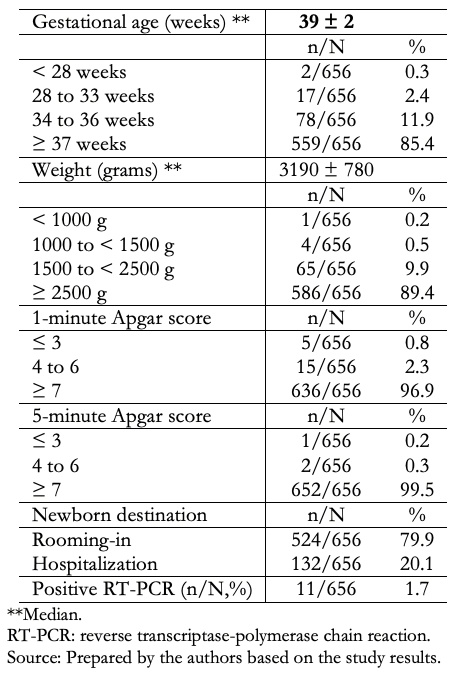 Full size
Full size Table 2 shows that 132/656 cases were hospitalized. The most frequent morbidities were neonatal sepsis (48.5%), hyaline membrane disease (9.1%), and jaundice (9.1%). A total of 99.4% of neonates were discharged.
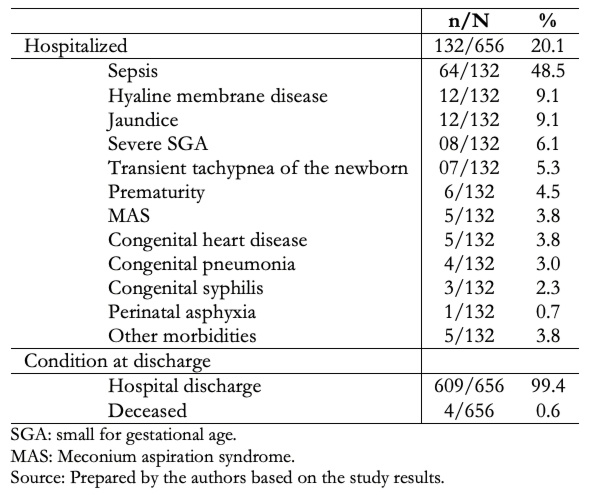 Full size
Full size Table 3 shows that the neonatal mortality was 4/656 (0.6%), where 75% and 25% had early and late neonatal deaths, respectively. The average birth weight was 1105 grams, with a range between 720 and 1600 grams. One-third and two-thirds of early neonatal deaths were due to extreme immaturity and hyaline membrane disease, respectively. The only late neonatal death occurred as a consequence of necrotizing enterocolitis.
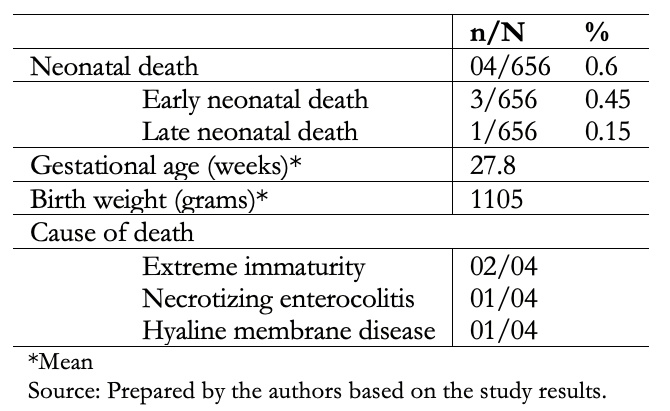 Full size
Full size Table 4 describes the clinical and laboratory characteristics of the 647 mothers included in the study. We found that 4.3% of mothers were symptomatic, 71.4% had mild, 17.9% moderate, and 10.7% severe symptomatology. There were no maternal deaths. Obstetric complications occurred in 27.4% of the pregnant women, the most frequent being preeclampsia 76/177 (42.9%), premature rupture of membranes 48/177 (27.1%), and gestation-induced hypertension 15/177 (8.5%). All were serologically tested for antibodies to SARS-CoV-2, with IgM(+) IgG(-) observed in 7.4%, IgM(+) IgG(+) in 81.9% and IgM(-) IgG(+) in 10.7%.
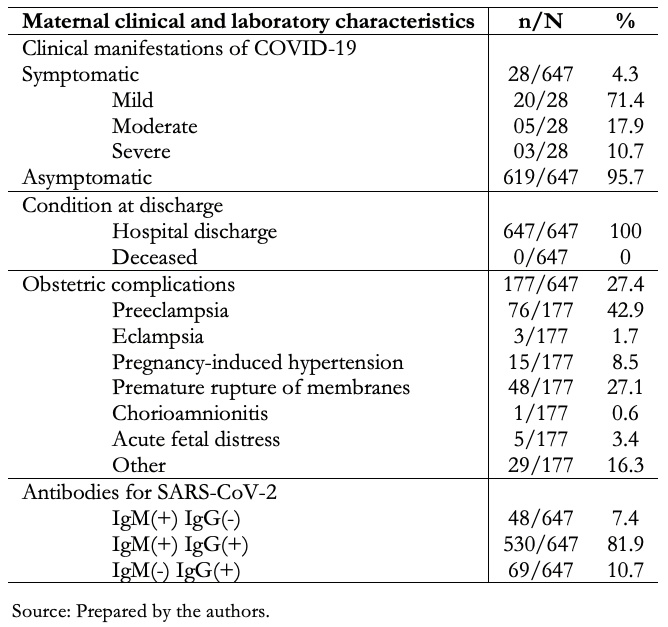 Full size
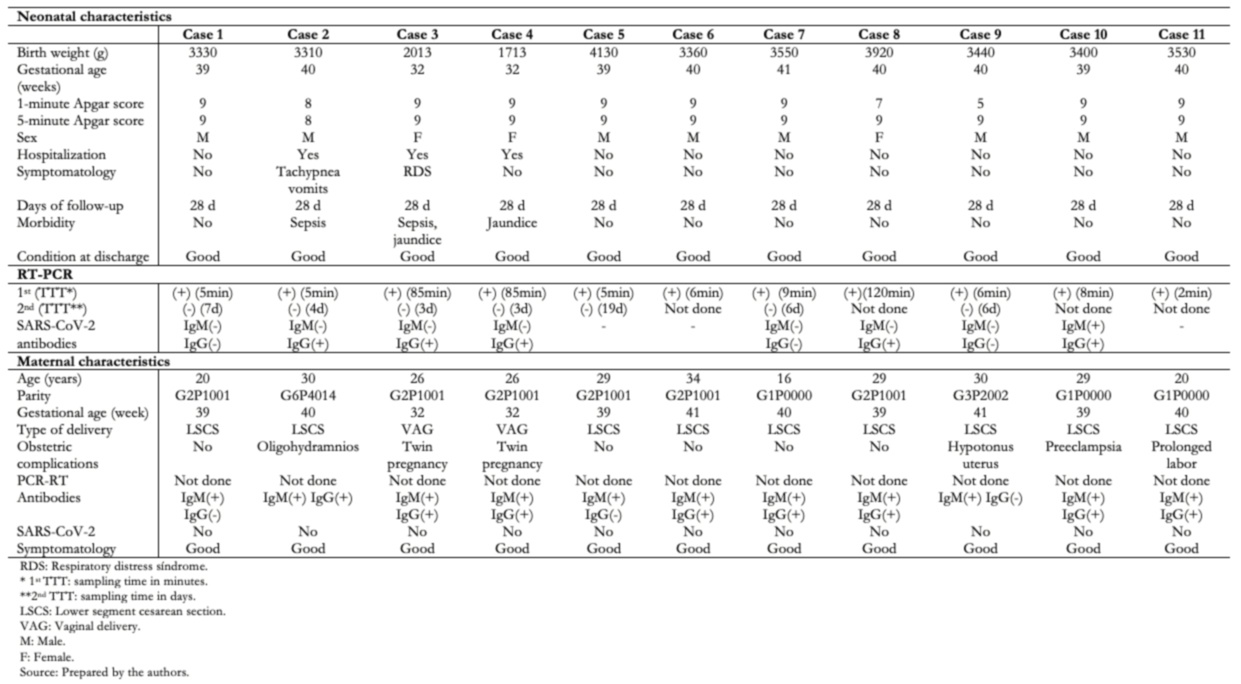
Full size Table 5 shows the clinical, epidemiological, and laboratory characteristics of the 11 neonates who had positive RT-PCR for SARS-CoV-2. Of these, three (27.3%) cases were hospitalized, two (18.2%) presented clinical manifestations such as respiratory distress, tachypnea, vomiting, and one (9.1%) jaundice. All cases were discharged in good condition and followed up by telephone 28 days after birth, and none presented complications.
 Full size
Full size Discussion
Viral infections are frequent complications in pregnancy, and COVID-19 can cause infections in pregnant women ranging from asymptomatic to a very severe disease that can lead to critical conditions and even death. Many microorganisms have vertical transmission within the prenatal period (congenital infection), at or around the time of delivery (perinatal infection), as well as horizontal transmission (postnatal infection), with a wide range of obstetric and neonatal sequelae. During the prenatal period, an infection can occur at any time during gestation. The consequences in the fetus can range from embryopathy (if the infection is in the first trimester of gestation), fetal infection in the second trimester, and symptoms caused by the immune response in the second and third trimester [31][32].
The risk of vertical transmission during vaginal delivery in pregnant patients with COVID-19 is still a matter of debate. As long as we are unclear about the mechanism of transmission, obstetric guidelines should be promoted to reduce the potential risk of perinatal infection during vaginal delivery [33].
In this study, most neonates were term newborns, had a birth weight equal to or greater than 2500 grams, and had 1-minute and 5-minute Apgar scores equal to or greater than seven. These results differ from Bwire and collaborators [34], who found in a systematic review that 62.5% of mothers with COVID-19 had preterm births, while 37.5% had term births. Interestingly, 3.2% of term and 18.4% of preterm newborns were positive for the SARS-CoV-2 virus. Moreover, Zeng et al. [18] and Neef et al. [35] reported that neonates born to mothers with COVID-19 infection had 1-minute Apgar scores of seven to 10 and 5-minute Apgar scores of eight to 10.
Of all newborns, 20.1% were hospitalized, and the remaining 79.9% stayed in a room with their mothers – maintaining biosecurity measures, such as a distance of two meters between the mother and baby and a permanent use mask by the mother. These results agree with Neef and collaborators [35], who report that of 261 neonates born to mothers with COVID-19, 80.4% were asymptomatic, and the remaining percent had mild symptoms. The latter findings could be because the population mainly consisted of term newborns, who usually have fewer complications in the neonatal period.
All neonates underwent nasopharyngeal swab for RT-PCR for SARS-CoV-2 within 2 hours of birth, and 1.7% of tests returned positive. Neef et al. [35] conducted a review study involving 261 newborns born to mothers with COVID-19, and 10% were positive for SARS-CoV-2. Bwire et al. [34], in a systematic review of 33 articles, included 205 infants born to COVID-19 positive mothers, reporting that 6.3% of the infants were positive for SARS-CoV- 2 at birth. In a review article, Barrero et al. [36] cite a cohort study conducted in the United Kingdom by Knight, finding a 5% prevalence of positive newborns born to SARS-CoV-2 infected mothers. Also, the latter review article cites a Spanish cohort by Martinez-Perez where perinatal infection occurred in 6, 9% of exposed newborns. Data from the National Surveillance and Epidemiology Registry for Perinatal COVID-19 Infection (NPC-19) in the United States report that 1.9% of viral tests were positive in newborns born to mothers with confirmed SARS-CoV-2 infection. Shalish et al. [37] conducted a review study of 137 newborns born to mothers with COVID-19 and found that 3% had positive RT-PCR tests for SARS-CoV-2. In all, these studies had 1.9 to 10% RT-PCR positivity in infants born to mothers with COVID-19, which is higher than our results.
Of all neonates studied, 20.1% were hospitalized. The most frequent diagnoses were sepsis, hyaline membrane disease, jaundice, small for gestational age, and transient tachypnea of the newborn. These findings agree with Neef and collaborators [35], who report that 82.5% of neonates were discharged without complications, supporting this population's mild course of infection. Among the most frequent symptoms, they report dyspnea (42.3%), fever (19.1%), vomiting (16.1%), and small for gestational age (8.1%). Similarly, Zeng et al. [38], in a cohort study of 33 newborns born to mothers with COVID-19, 3 (9%) newborns were positive for SARS-CoV-2, their clinical symptoms were mild, and outcomes were favorable. However, the results differ from those reported by Liguoro et al. [39], who performed a systematic review that included 25 newborns with SARS-CoV-2. Of these, 20% were asymptomatic, 48% had mild signs of clinical infection, 20% had moderate symptoms, and 12% were severely ill. Dyspnea was the most common sign at neonatal age (40%), but fever (32%) and feeding intolerance (24%) were also described.
We observed that mortality was 0.6%, and none of the deaths were attributed to COVID-19. Of the 11 neonates who had positive RT-PCR for SARS-CoV-2, 27.3% were hospitalized for clinical manifestations such as respiratory distress, tachypnea, vomiting, and jaundice. All cases were discharged in good condition and were followed up by telephone until 28 days after birth, and none of them presented complications. These results are in line with Neef and colleagues [35], who report that 66.7% of neonates who tested positive for SARS-CoV-2 had mild clinical symptoms and that the onset of symptoms was before three days of birth. They also reported 1.7% of perinatal deaths. These included a 38-week gestational age stillborn case with severe chronic villitis, a 35-week gestational age preterm neonate with severe asphyxia, and a 34-week gestational age preterm neonate with late neonatal death with multiorgan failure disseminated intravascular coagulation, and refractory shock. The SARS-CoV-2 infection could not be demonstrated in all cases. Available information shows that neonates usually have mild clinical symptoms, but severe symptomatology could be present in some cases. COVID-19 is not reported as a cause of neonatal death.
In our study, the mothers presented COVID-19 in the third trimester of gestation, 95.7% were asymptomatic, and none died. Barrero and collaborators [36] report that infection rates in pregnant women fluctuate according to the regions. A health facility in New York City reported that 20% of pregnant women tested positive for SARS-CoV-2, and 13% were asymptomatic; in contrast with a study in Connecticut that reported that 3.9% of pregnant women tested positive for SARS-CoV-2 and 2.9% of them were asymptomatic. Likewise, Yang et al. [12] reported that COVID-19 disease in pregnant women to be 86% mild, 9.3% severe, and 4.7% critical, similar to non-pregnant adult women. Blitz et al. [40] report that of all women hospitalized for COVID-19, pregnant women did not have a higher risk of admission to the intensive care unit compared to non-pregnant women. In contrast, Hantoushzadeh et al. [41] report nine pregnant women diagnosed with SARS-CoV-2 infection, all of whom presented severe clinical symptoms, 7/9 (77.8%) had died, 1/9 remained in critical condition and ventilator-dependent, and 1/9 were in recovery. Our findings contrast with those reports since there was no mortality in pregnant women affected by COVID-19. The differences could be explained because the mothers included in this study presented COVID-19 in the third trimester, and most were asymptomatic.
The most frequent obstetric complications were preeclampsia, premature rupture of membranes, and gestational hypertension. Yu and colleagues [16] reported seven pregnant women with COVID-19 that ended in delivery, none of them presented obstetric complications, and all had mild clinical symptoms. On the other hand, Yan and colleagues [15] reported that 6/99 (6.1%) patients presented premature rupture of membranes, and 21/99 (21.2%) had preterm delivery. Liu and colleagues [13] studied 13 pregnant patients with COVID-19, five of them ended in emergency cesarean delivery, and six had preterm labor. The obstetric and perinatal complications could be attributed to SARS-CoV-2 infection in addition to the physiological changes during gestation, making the woman less tolerant to hypoxia during the third trimester of pregnancy.
The 11 neonates with positive RT-PCR for SARS-CoV-2 had nasopharyngeal swab samples taken between five and 120 minutes after birth. Their mothers had positive immunoglobulin tests for SARS-CoV-2 with positive IgM, and all were asymptomatic. To diagnose SARS-CoV-2 infection in symptomatic pregnant women, the virus must be detected by RT-PCR (in a respiratory specimen); there must also be a history of positive epidemiological contact in asymptomatic women. In neonates without clinical signs of infection and born to infected mothers, a nasopharyngeal RT-PCR positive for SARS-CoV-2 should be taken at birth, but a second negative sample corresponds to a case of possible intrapartum-acquired neonatal infection [42].
One of the most important limitations of the study was the scarcity of molecular tests because public health facilities in our country are on restricted budgets. Since it was not possible to perform RT-PCR nasopharyngeal samples or take samples from the placenta, amniotic fluid, or umbilical cord blood, we could not confirm COVID-19 diagnoses. Therefore, vertical transmission could not be demonstrated. According to the definition of cases in pregnant women and neonates published by Shah and collaborators [42], we could say that we are facing cases of possible intrapartum-acquired neonatal infection. However, due to the limitations in performing diagnostic tests, we cannot exclude the possibility of vertical transmission in the reported cases.
The possibility of transmission of SARS-CoV-2 from mother to fetus is a current concern, and there are several studies on the subject. A mother could become infected at any time during pregnancy. In addition, the outcomes in the fetus when maternal infection occurs early in pregnancy may be different than when it occurs close to delivery. Infection during the first or second trimester could cause miscarriage, premature delivery, birth defects, or possibly other features of congenital infections. However, in maternal infection near delivery, we must consider the possibility that the newborn may have an active infection [42].
Pulinx et al. [32] and Penfield et al. [43] report cases in which they tested for SARS-CoV-2 in placental tissue, membranes, and amniotic fluid with positive results. Algarroba et al. [44] reported a woman of 28 weeks of gestation with severe COVID-19. The placenta was studied using electron microscopy to evaluate possible vertical transmission. In these studies, coronavirus virions invading syncytiotrophoblasts were visualized in placental villi. In addition, SARS-CoV-2 virions were identified in the cellular processes of placental villus fibroblasts, and the infant tested negative for COVID-19 on days two and three of life. These findings contribute to evidence of placental infection with SARS-CoV-2. However, there was no evidence of fetal infection.
Vivanti et al. [45] and Zamaniyan et al. [46] reported cases of amniotic fluid samples taken before rupture of membranes testing positive for SARS-CoV-2. The neonates in both studies had nasopharyngeal swab samples positive for SARS-CoV-2, suggesting a probable intrauterine infection with a transplacental transmission.
Vertical transmission of SARS-CoV-2 could occur through breast milk, which is still highly debated. Some studies have been found reporting the presence of the virus in breast milk. However, it has not been possible to confirm this mechanism of infection. Grob et al. [47] and Bastug et al. [48] report cases of mothers with COVID-19 who had breast milk samples positive for SARS-CoV-2, the offspring of both women tested positive for SARS-Cov-2, but it was not possible to confirm whether the neonates were infected through breast milk. On the other hand, Buonsenso et al. [49] reported the case of a SARS-CoV-2-positive mother whose breast milk was collected for RT-PCR during the first five days postpartum that tested positive in 3/5 samples for SARS-CoV-2. Meanwhile, the neonate underwent RT-PCR for SARS-CoV-2 in nasopharyngeal and rectal swabs, and all results were negative.
Previous studies are inconclusive regarding the vertical transmission of SARS-CoV-2 through breast milk, so we must evaluate the importance of continued breastfeeding. In addition to the well-known benefits of breastfeeding, it has been possible to isolate specific IgG and IgA antibodies against SARS-CoV-2 in human milk, which have shown a strong neutralizing capacity for SARS-CoV-2. While these data show a robust immune response of breast milk against the virus, they also suggest that breast milk provides an active form of protection against the virus, which supports the protection and promotion of breastfeeding [50][51].
According to the reported studies, there is a possibility of vertical transplacental or breast milk transmission. However, there is still no explanation as to why some neonates are affected and others are not.
Similar to other viral infections, pregnant women are vulnerable to SARS-CoV-2 disease. Although current reports show a low incidence of vertical transmission, further studies are needed. Also, when a maternal infection is acquired in the last weeks of gestation, the neonatal disease is usually mild to moderate. However, more evidence is still lacking on the risks to the fetus and neonate when COVID-19 infections occur in pregnant women during the first trimester.
Conclusions
Of all neonates studied, 1.7% had a positive RT-PCR test for SARS-CoV-2, 14.6% were preterm newborns, 20.1% were hospitalized, and the most frequent morbidity was neonatal sepsis. Mortality was 0.6%, with no cases attributed to COVID-19. The neonates with positive SARS-CoV-2 who were hospitalized presented a mild disease.
More than 95% of the pregnant women with COVID-19 were asymptomatic. Of those who presented clinical symptoms, most were mild to moderate. There was no record of maternal mortality in the study group.
The limited availability of diagnostic tests does not allow us to assess the possibility of vertical transmission of SARS-CoV-2 or cases of neonatal infection acquired intrapartum.

