Health economics
← vista completaPublished on March 15, 2022 | http://doi.org/10.5867/medwave.2022.02.002118
Cost-effectiveness study of prophylaxis with emicizumab versus bypassing agents in patients with severe hemophilia A in Peru
Estudio de costo-efectividad de profilaxis con emicizumab versus agentes hemostáticos alternativos en pacientes con hemofilia A grave en Perú
Abstract
Settings Hemophilia is a coagulation disorder that occurs in one in 5000 male births. Patients with untreated severe hemophilia A have hemorrhagic complications, including joint bleeds and decreased survival. Emicizumab is a monoclonal antibody approved by the United States for routine prophylaxis of pediatric and adult patients with severe hemophilia A with factor VIII inhibitors.
Objectives To perform a cost-effectiveness study of emicizumab prophylaxis for children and adults with severe hemophilia A compared with the current disease management in the Peruvian Ministry of Health and Social Security Health Insurance.
Methods The patient transition between medical states was modeled with Markov methodology, and the lifetime costs and incremental effects of emicizumab compared to current management were estimated. The budgetary impact of emicizumab was estimated by projecting annual net costs and its five-year present value.
Results In the Ministry of Health, emicizumab would generate savings between 14.6 and 16.0 per child and 11.8 per adult, in current US$ million. Social Security Health Insurance savings would be 12.8 to 14.9 per child and 40.1 per adult. In addition, this strategy would generate effectiveness gains, measured in quality-adjusted life-years, of 0.36 per child and 0.56 per adult and 0.25 per child, and 0.36 per adult in those respective institutions. The budgetary impact would be a net annual saving of 12.8 and 15.0 US$ million in those entities.
Conclusions The current management of hemophilia A is very costly and has health outcomes inferior to those possible with emicizumab. This drug would produce significant savings and better patient health. The Ministry of Health and Social Health Insurance should implement hemophilia prophylaxis and treatment protocols and finance this drug.
|
Main messages
|
Introduction
Hemophilia is a generally hereditary bleeding disorder caused by problems in blood coagulation. Hemophilia A accounts for 85% of all cases and is generated by insufficiency of coagulation factor VIII.
About 440 000 people have severe hemophilia A worldwide [1]. Untreated patients have significant bleeding complications, including joint bleeds and decreased survival, with an average life expectancy of 8 to 11 years. In the United States in 2017, the burden of disease caused by severe hemophilia A was estimated to be
0.33 disability-adjusted life-years per 1000 population. Co-authors of this article estimated that there are currently 3000 people with hemophilia in Peru. Of those, only 1002 have an official diagnosis, and two-thirds have a severe form of hemophilia. In 2016, Peruvians would have lost 168.8 disability-adjusted life years per 1000 population due to all causes [2]. Extrapolating the United States (US) ratio, hemophilia would cause in Peru just 0.20% of the US disease burden, although it imposes a much higher percentage expenditure on the health system.
Historically, hemophilia has been treated by periodic transfusion of clotting factors derived from blood plasma or manufactured by genetic recombination. Unfortunately, 5-7% of patients with hemophilia A and one-third of patients with severe hemophilia A develop antibodies that inhibit clotting factors and increase mortality. Patients with high levels of inhibitors are treated with alternative hemostatic agents, such as activated prothrombin complex concentrate or recombinant factor VIIa. Children diagnosed with severe hemophilia A may undergo immunotolerance with the administration of high doses of factor VIII to decrease inhibitor antibodies production. This latter procedure only benefits 60-80% of patients under 18 years of age [3].
Severe hemophilia A is one of the most expensive diseases to treat. In the United States, treating one bleeding episode can cost US$ 50000. Patients with severe hemophilia A receive intravenous factor VIII concentrates several times a week to reduce bleeding. Because of its high cost (between US$ 300 000 and US$ 2.5 million per patient annually), only a few receive prophylaxis with alternative hemostatic agents [4].
A worldwide consensus favors prophylaxis over bleeding treatment with the intermittent infusion of factors to prevent spontaneous bleeding. Still, the Ministry of Health in Peru does not provide prophylaxis for severe hemophilia A and treats bleeds with anti-inhibitor coagulant complex or recombinant activated factor VII donated, according to availability. Within the Social Security Health Insurance, all children with inhibitors receive immunotolerance and prophylaxis with anti-inhibitor coagulant complex. Adults do not receive prophylaxis: their bleeding episodes are mainly treated with anti-inhibitor coagulant complex (90%) or by a second line with activated factor VII (10%).
The monoclonal antibody emicizumab (Hemlibra®), approved in 2017 by the Food and Drug Administration, functions as a cofactor for factor VIII. In patients on inhibitors, prophylaxis with emicizumab showed an 87% reduction in bleeding compared with patients without prophylaxis. Two-thirds of patients on prophylaxis were free of bleeding during one year. In patients without inhibitors, the effects were even more favorable. Weekly subcutaneous application of emicizumab facilitates patient adherence, reduces bleeding and hospitalizations, and improves the quality of life. The current evidence recommends that emicizumab should be indicated for hemophilia A prophylaxis with and without inhibitors. However, such a decision should be based on a cost-effectiveness economic evaluation to determine whether the incremental health benefits associated with the use of emicizumab justify its incremental cost.
This study sought to determine the cost-effectiveness of emicizumab as prophylaxis for patients with severe hemophilia A. This treatment was compared with current therapeutic alternative schemes in Peru for adults and children covered by the Ministry of Health and Social Security Health Insurance. Treatment alternatives for severe hemophilia A patients included prophylaxis (with anti-inhibitor coagulant complex or recombinant activated factor VII) or no prophylaxis.
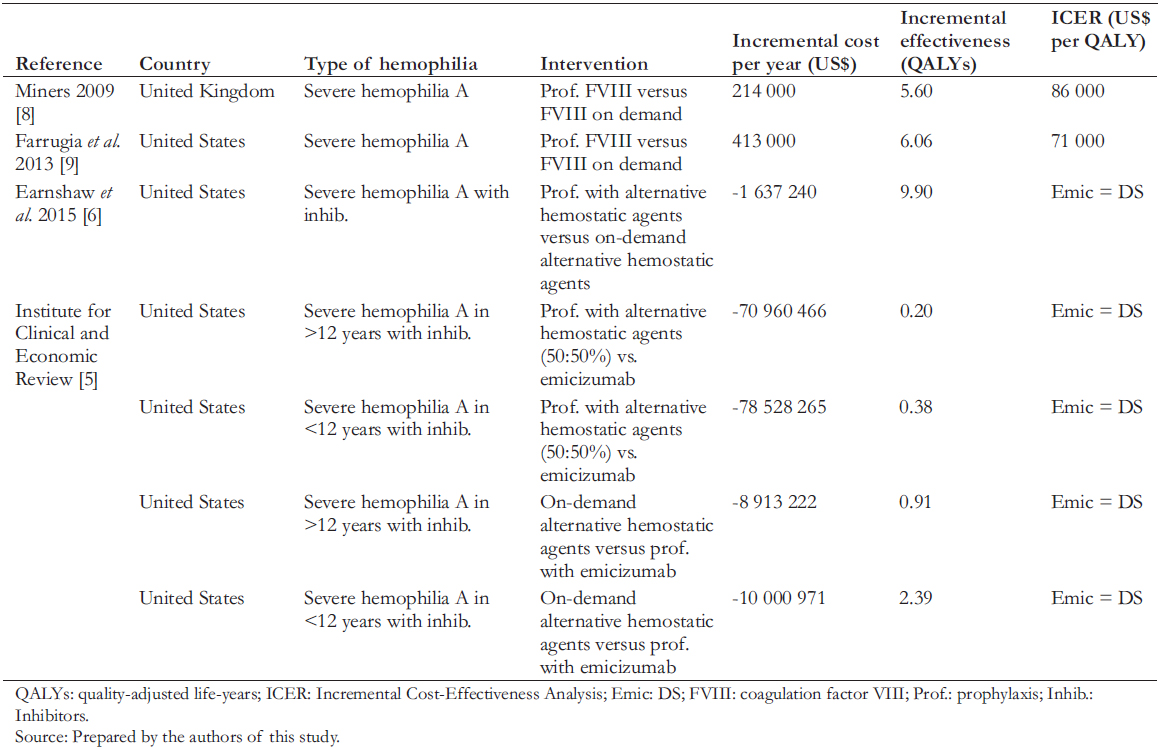
Few international cost-effectiveness studies compare prophylaxis with treating bleeding episodes for hemophilia. Table 1 shows these studies, with a summary of the literature review. The evidence shows incremental cost-effectiveness ratios within acceptable ranges for incorporating new technology into their respective countries' financing or insurance regime and shows a reduction in disability and increased patient productivity. Only one study with inhibitors in prophylaxis compares the use of this new drug in patients with prophylaxis and alternative hemostatic agents or with on-demand treatment [5]. It concluded that emicizumab reduces annual medical costs per patient by US$ 1.9 million among patients older than 12 years and by US$ 720,000 in younger than 12 years. It also found that emicizumab prophylaxis compared with on-demand treatment or prophylaxis with alternative hemostatic agents could be costsaving. In addition, emicizumab is more effective and allows a lower cost reduction in disease burden than the other management strategies.
 Full size
Full size Methods
Design
A cost-effectiveness analysis was performed from a payers perspective (Ministry of Health or Social Security Health Insurance), using a model that simulates the transition of patients between different medical states using the Markov methodology. The model was implemented with the TreeAge program to simulate the natural history of a hemophilia patient in Peru.
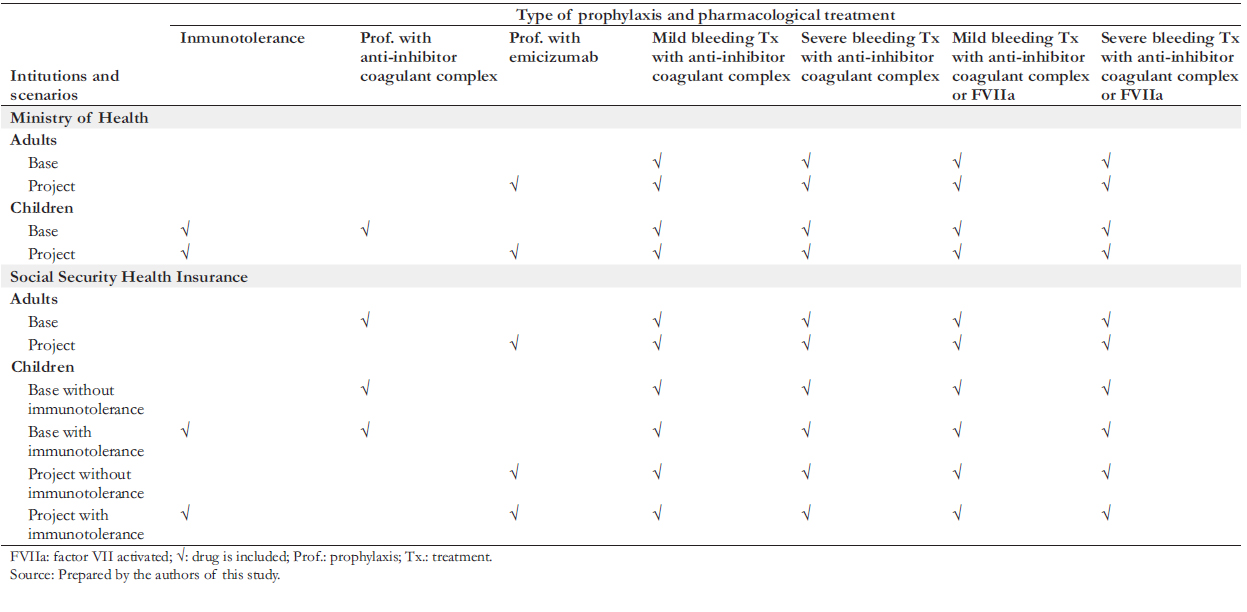
Two scenarios were formulated: the "base scenario", which represents the current situation in the Ministry of Health and the Social Health Insurance, and the "project scenario", which represents the adoption of emicizumab prophylaxis in the two institutions. In both scenarios, children (under 15 years of age) in the Ministry of Health and the Social Security Health Insurance receive prophylaxis. This strategy is done with an anti-inhibitor coagulant complex in the base scenario and emicizumab in the project scenario. Likewise, adults covered by the Ministry of Health do not receive prophylaxis in any scenarios. In the Social Security Health Insurance, the base scenario contemplates that adults receive prophylaxis with anti-inhibitor coagulant complex, while in the project scenario, this is done with emicizumab. Additionally, both scenarios contemplate performing immunotolerance to children that present inhibitors in some instances. Table 2 summarizes the design elements described above.
A budget impact analysis was also performed for the Ministry of Health and the Social Security Health Insurance. i.e., the additional annual cost associated with emicizumab as a prophylactic scheme in these two institutions. For this purpose, the costs per patient were multiplied by the annual number of patients who would use emicizumab for severe hemophilia A prophylaxis. It was assumed that initiation of emicizumab prophylaxis occurs at two years in children and 18 years in adults. The horizon of the cost-effectiveness study was 16 years for children and 52 years for adults. The horizon of the budget impact analysis was five years. A literature review concludes a lack of knowledge regarding the annual number of bleeds in patients with severe hemophilia A [5]. Therefore, the Institute for Clinical and Economic Review performed simulations on the number of bleeds [5]. The authors obtained an estimate of the average number of annual bleeds in children and adult patients from Peruvian experts to fill this gap. Although the literature reports an average duration of immunotolerance of
26.2 months until complete tolerance is achieved, the national experts reported that this usually lasts nine months in Peru. Since the dosage of emicizumab is based on the patient’s weight, in consultation with the experts, we assumed that the average weight of a child is 35 kilograms and 70 kilograms for adults. We also specified the posology by noting the dose and frequency of administration (Table 3).
Costs
To define the characteristics of prophylaxis, doses, amount of bleeding, and types of treatment of critically ill patients, we collaborated with a committee of Peruvian experts in hemophilia, all of whom participated as co-authors of this article. The estimated annual costs of drugs consumed by patients with severe hemophilia A are shown in Table 3. The emicizumab costs considered the current selling price in addition to a simulation with a 20% discount. The Roche laboratory in Peru provided both the price and the discount. We considered nine months of use to estimate immunotolerance costs in children. For the costs associated with the care received during bleeding states (mild or severe), the price lists of two hospitals with experience in treating hemophilia were analyzed: the Hospital Nacional Dos de Mayo and the Clínica Ricardo Palma. Considering that the values of public hospitals are heavily subsidized, it was decided to base prices from Clínica Ricardo Palma, a private establishment, since the latter would be closer to the actual cost (Table 4). In addition, a simulation was carried out using the costs of the Hospital Nacional Dos de Mayo.
Concerning prophylaxis and bleeding treatments, we included drug costs only with the information provided by hematologists specialized in hemophilia and considered an efficient use of emicizumab, taking into account its presentation. We did not include costs of human health resources, such as physicians, nurses, and assistants, because we consider them marginal compared to drug costs. This consideration aligns with published literature (e.g., Earnshaw et al. [6]). The annual costs of caring for patients with severe hemophilia A by an institution are presented in Table 3. The pharmacological components are specified in Table 4. All monetary figures were expressed in soles and US dollars on December 31, 2019.
Effectiveness
To be consistent with the pharmacoeconomic evidence on severe hemophilia A, we measured effectiveness in terms of gains in quality-adjusted life-years. Since no information on this measure is available in Peru, we used quality-adjusted life-years reported by the Institute for Clinical and Economic Review [5] for the health states that noted: ’severe bleeding' 0.54, 'mild bleeding' 0.66, 'no bleeding' 0.82 [5].
Pharmacoeconomic evaluation
Using the Markov methodology, the formulated model simulated how hemophilia patients transit with different probabilities (Table 5) between four different health states (Figure 1) in consecutive one-week cycles over time. In each of the four states (e.g., "no bleeding"), the patient has a certain probability of remaining in that state until the next cycle, and three other probabilities of changing state during the cycle (e.g., "death," "mild bleeding," or "severe bleeding").
We considered two scenarios: the base scenario, which corresponds to the current management of hemophilia patients in the Ministry of Health and the Social Security Health Insurance; and the project scenario, which considers emicizumab prophylaxis for three types of patients: adults with inhibitors, children with inhibitors and no immunotolerance, and children with inhibitors and immunotolerance. This event analysis aligns with the Institute for Clinical and Economic Review [5] in their costeffectiveness study of emicizumab [5]. The time horizon for adults was 52 years (equivalent to the life expectancy for hemophilia A and B patients reported in an English study) and 16 years for children [7]. The model implemented in the TreeAge computer program considered four excludable states: no bleeding, mild bleeding, severe bleeding, and death; and eight transition probabilities.
Both costs and effectiveness in future years were discounted to present value, using a real annual discount rate of 3% consistent with the only other cost-effectiveness analysis published from the payer’s perspective [5].
The values resulting from the Markov model (emicizumab prophylaxis scenarios) were compared with their respective base scenarios for the Ministry of Health and the Social Security Health Insurance. Thus, three comparative pairs were formed (base scenario versus project scenario) for the Ministry of Health and three for the Social Security Health Insurance: one for adults with inhibitors, another for children with inhibitors without immunotolerance, and another for children with inhibitors with immunotolerance. The following assumptions were taken into account for the construction of the model: a) Patients with severe hemophilia A (less than 1% of standard clotting factor VIII). b) The average age of initiation of emicizumab prophylaxis in children is two years and in adults 18 years. c) The number of annual bleeding episodes in the baseline scenario for the Ministry of Health was eight, and for the Social Security Health Insurance was six. d) The number of annual bleeding episodes in the project scenario (in the Ministry of Health and the Social Security of Health Insurance) was one in children with inhibitors with and without immunotolerance and two in adults with inhibitors. e) In cases of bleeding, the additional costs of bed days, examinations, and follow-up medical consultations were considered. f) All cases that presented bleeding were treated with alternative hemostatic agents (current scheme).
For evaluating results, we calculated the present values of total costs; incremental costs (comparing the base and project scenario); effectiveness in quality-adjusted life-years; incremental effectiveness in quality-adjusted life years; and the incremental cost-effectiveness ratio in US dollars per quality-adjusted lifeyears. This ratio is interpreted according to its location in the quadrants of Figure 2.
The new technology improves the patient’s health status and reduces costs in the ideal case. This option is called the "dominant" strategy because it is unambiguously desirable. The undesirable case (when it decreases health status and increases costs) is the "dominated" strategy. Cases in which the new technology improves health status but increases costs or worsens health status but decreases costs should be compared with other medical interventions to determine their relative merit.
Finally, simulations were performed with the model considering reductions in the price of emicizumab.
Budget Impact analysis
The present value of the incremental cost for the project scenario represents the savings or increased expenditure (negative or positive incremental cost, respectively) of the total possible cycles for a patient. This present value considered a weekly cost of prophylaxis of US$ 830 for a child and US$ 2707 for an adult. These values, multiplied by the number of child and adult patients, yielded estimates of the annual savings or increased expenditure and the present value over a five-year horizon. This value represents the impact on the budget currently allocated by the Ministry of Health or Social Security Health Insurance to adopt a prophylaxis policy with emicizumab. The impact would be favorable if this new policy would generate budgetary savings or unfavorable if it would require a larger budget. Authorities with decision-making power to adopt emicizumab prophylaxis need to know these results.
In the budget impact analysis we also consider a reduction in the number of future hospitalizations and in their respective costs, but we do not consider adverse events associated with the use of emicizumab since these are infrequent and with relatively minor consequences in the health of the patient.
 Full size
Full size 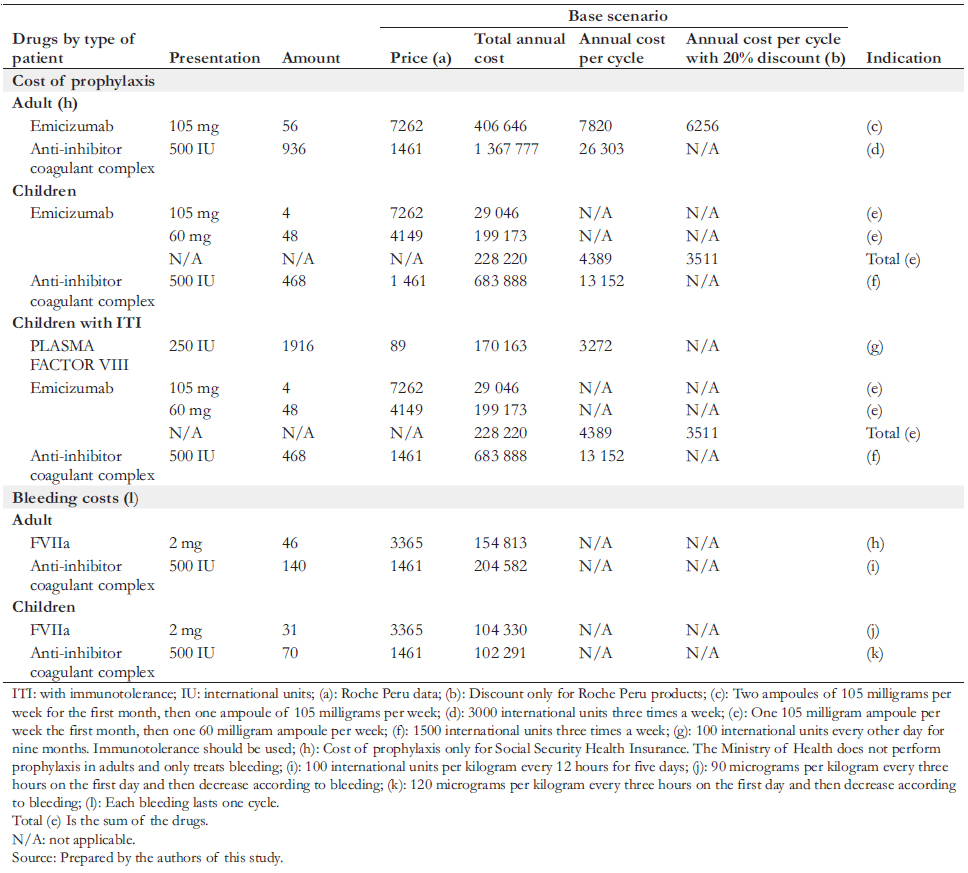 Full size
Full size 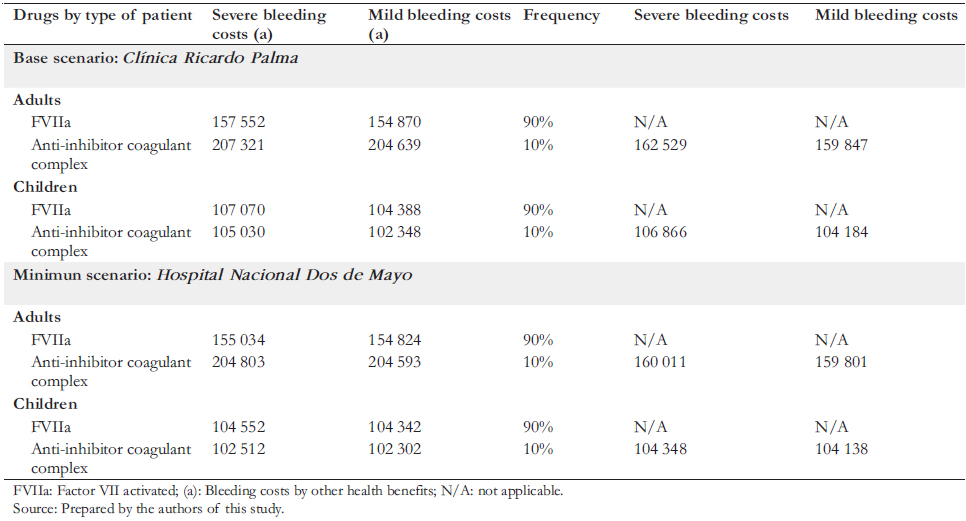 Full size
Full size 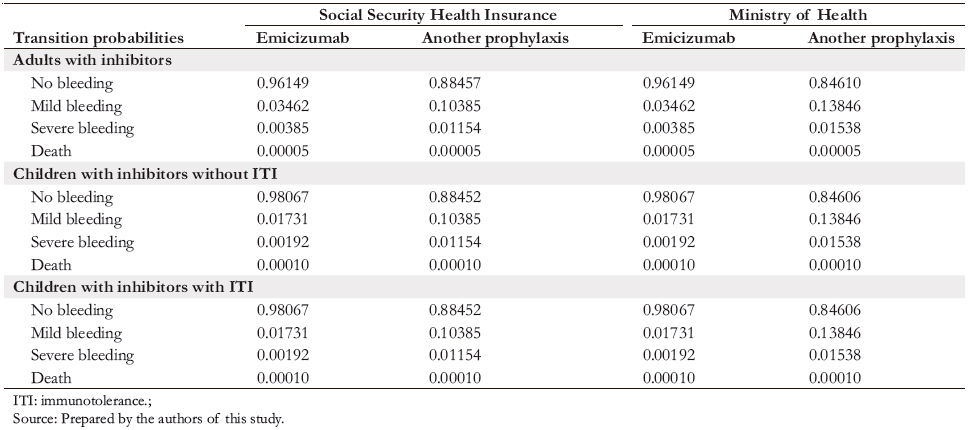 Full size
Full size 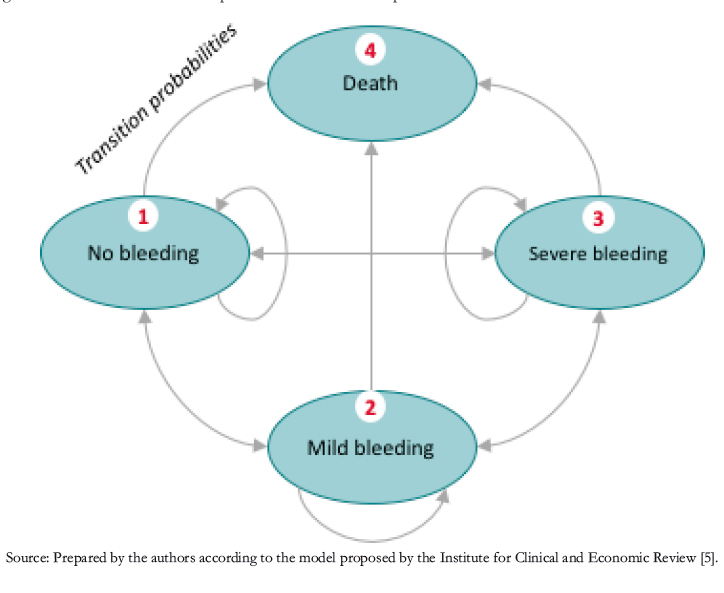 Full size
Full size  Full size
Full size Results
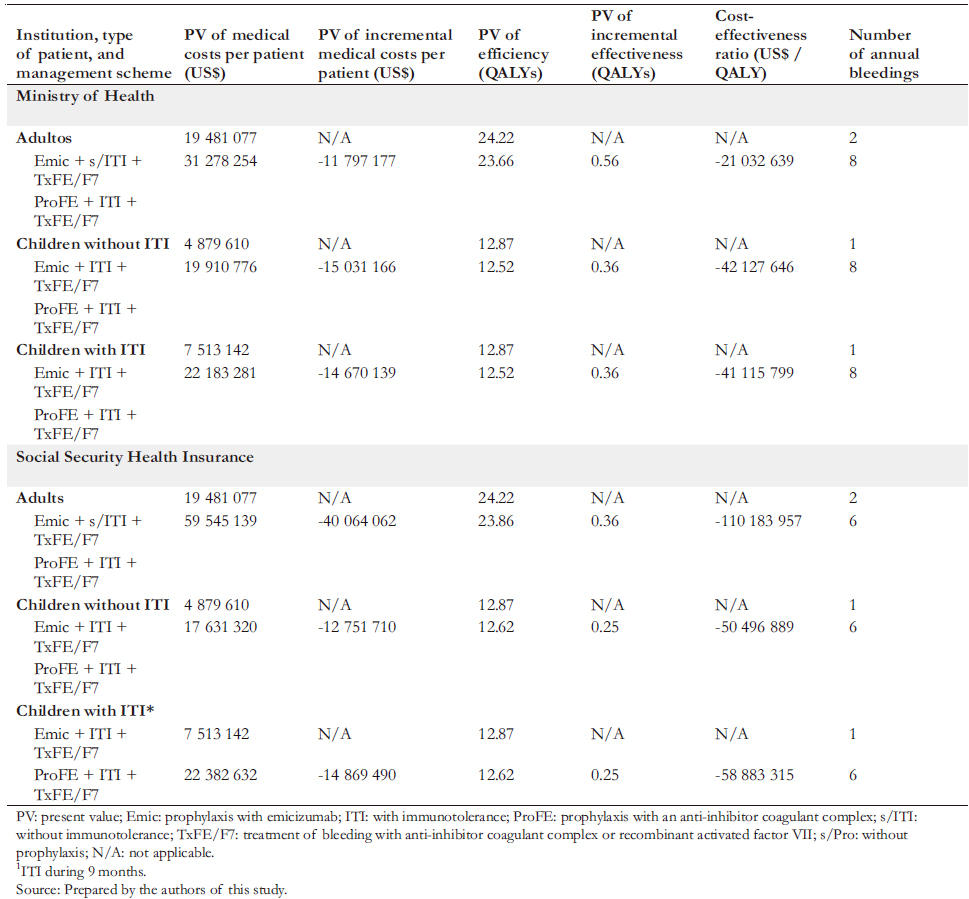
In both the Ministry of Health and the Social Security Health Insurance, we found that emicizumab proved to be the dominant strategy. In contrast, the dominated alternative strategies included prophylaxis with anti-inhibitor coagulant complex in children and adults, with or without immunotolerance (Table 6).
Compared to the current Ministry of Health strategy, prophylaxis with emicizumab would generate per patient US$ 42.1 million savings for children with inhibitors without immunotolerance, US$ 41.1 million for children with inhibitors and with immunotolerance, and US$ 21.0 million for adults with inhibitors. Moreover, emicizumab would also generate savings in the Social Security Health Insurance. These would be US$ 50.5 million for children with inhibitors and without immunotolerance, US$ 58.9 million for children with inhibitors with immunotolerance, and US$ 11.2 million for adults with inhibitors.
In the Ministry of Health, prophylaxis with emicizumab would generate an effectiveness gain per patient compared to the current strategy. This gain would be 0.36 quality-adjusted life-years per child with inhibitors and without immunotolerance and
0.56 quality-adjusted life-years per adult with inhibitors. In the Social Security Health Insurance, prophylaxis with emicizumab would also generate effectiveness gains compared to the current strategy for managing patients with severe hemophilia A. This difference would be 0.25 quality-adjusted life-years per child with inhibitors with and without immunotolerance and 0.36 quality-adjusted life-years per adult with inhibitors.
Regarding the incremental cost-effectiveness analysis, emicizumab is a dominant strategy in the Ministry of Health and the Social Security Health Insurance since it increases effectiveness and reduces costs.
A univariate sensitivity analysis was performed, showing the results obtained by modifying each parameter between a minimum and a maximum value. This analysis showed that despite the patient’s age and the current type of treatment, the adoption of emicizumab generates savings and improvement in health status (Table 7).
A probabilistic sensitivity analysis was also performed (Figure 3). The results are presented graphically below for children. This analysis shows that in almost 100% of the simulations, emicizumab prophylaxis would be cost-effective compared to the other treatments, with a threshold of less than US$ 45 258.
 Full size
Full size 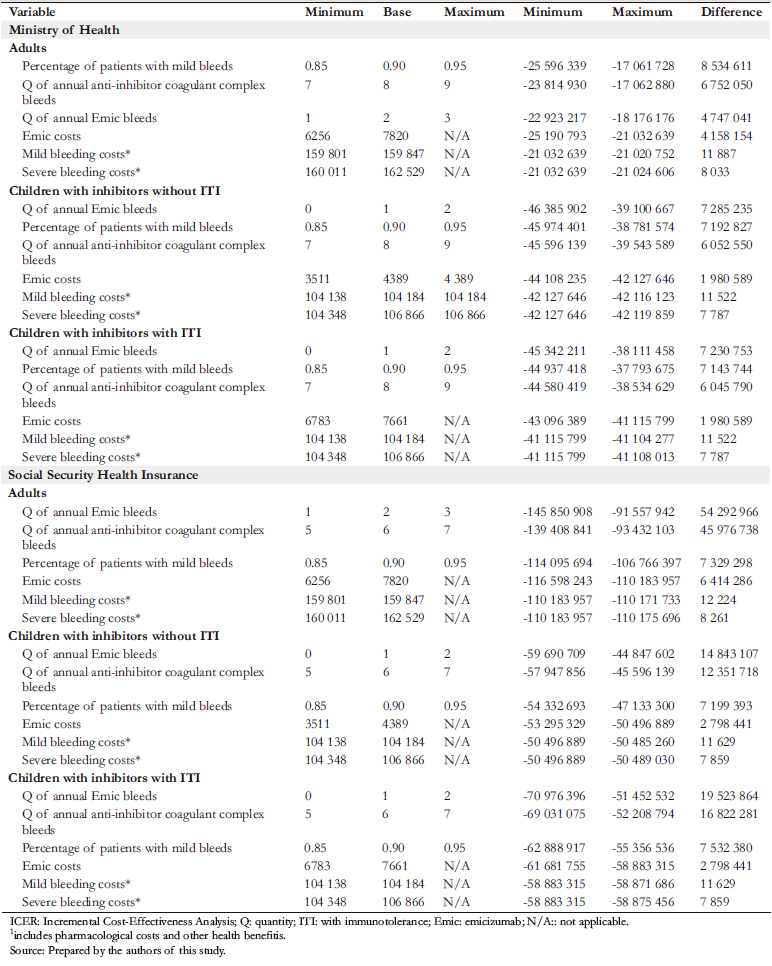 Full size
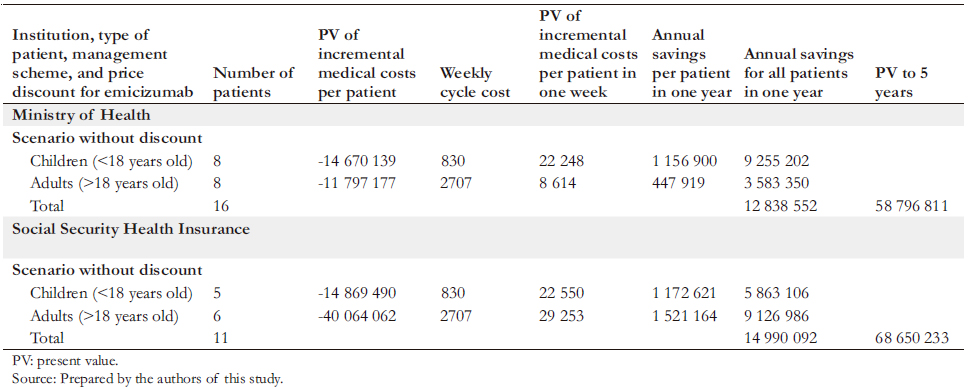
Full size The budget impact would be favorable for public finances. The adoption of emicizumab prophylaxis would result in significant net savings for the Ministry of Health and the Social Security Health Insurance (Table 8), producing an annual net savings of US$12.8 million in the former. In present value over five years, the savings would be US$ 58.8 million. In the Social Security Health Insurance, emicizumab prophylaxis would also result in considerable net savings: These would be US$ 15.0 million per year. Over five years, the present value of these savings would be US$ 68.7 million at the list price of emicizumab. In an alternative scenario – in which the price of emicizumab had a 20% discount (a possibility raised by Roche to the authors) – in five years, the savings for the Ministry of Health would be US$ 62.0 million and US$ 72.3 million for the Social Security Health Insurance.
At the time of writing, the Ministry of Health uses donations to finance treatment of bleeds in hemophilia patients. In addition, some patients may also have to make payments or copayments, the magnitude of which is not documented. Our budget impact analysis assumes that the Ministry of Health currently funds the drugs from its resources. However, if the funding comes in part from donations and patient payments, the budget impact of adopting emicizumab would be less (by an unknown amount) than what we estimate, at least for the Ministry of Health.
Discussion
The management of severe hemophilia A by treating bleeds "on-demand" generates enormous healthcare costs, causes severe disability in patients, and reduces their life expectancy. On the other hand, prophylaxis with an anti-inhibitor coagulant complex with and without immunotolerance is also very expensive. Similarly, its results on patient health are not as good as those obtained when prophylaxis is performed with emicizumab. The results of this study show that the two large public health institutions in Peru – which provide medical care to almost the entire population of the country – currently incur high costs in managing severe hemophilia A, while their patients unnecessarily experience a high burden of disease. They also show that emicizumab prophylaxis would generate considerable economic savings for these two institutions and improve the health status of severe hemophilia A patients. These results are consistent with other studies published in developed countries, as shown in Table 1.
The methodology adopted in this work, with the formulation of a Markov model that accounts for the life cycle of patients with severe hemophilia A and the different health states through which they pass, is consistent with other studies in this field, including the most recent one conducted by the Institute for Clinical and Economic Review. Moreover, this effort brought together some of Peru’s leading hemophilia experts to document (for the first time) the costs of managing severe hemophilia A through the Ministry of Health and the Social Security Health Insurance. These experts also contributed to estimating the gains in health status and medical expenditure that would result from emicizumab as prophylaxis for severe hemophilia A.
One of the uncertainties of this work stems from the lack of knowledge of the actual costs of healthcare in the Ministry of Health and Social Security Health Insurance hospitals. Using the costs of a private clinic as a proxy could overestimate the actual costs of these two institutions in managing hemophilia. However, simulations of healthcare costs show that even if public costs were substantially lower than private costs, the study’s main result would not change.
We should note that it is uncommon to obtain as a result of a cost-effectiveness study that new medical technology will reduce costs and improve the health status of patients. It is usual that for the burden of disease produced by a given pathology, the health system should incur a higher cost than current management. The fact that emicizumab prophylaxis is dominant speaks to the high convenience of adopting this treatment strategy.
 Full size
Full size 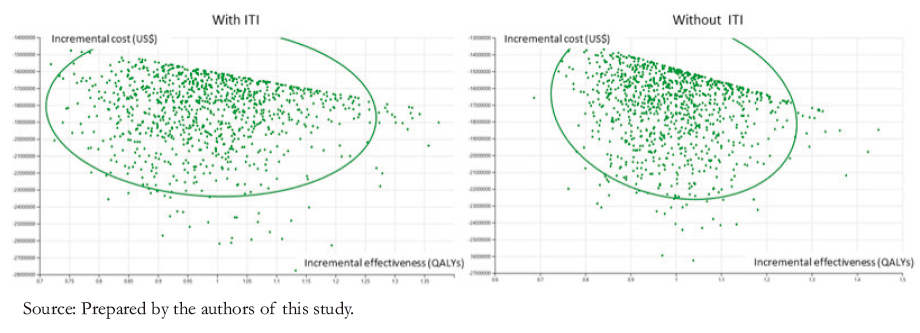 Full size
Full size Conclusion
This analysis has shown that emicizumab as prophylaxis for severe hemophilia A in children and adults covered by the Ministry of Health or the Social Security Health Insurance is a dominant cost-effective strategy: it provides better health outcomes at lower costs than the current therapeutic scheme. Its use would generate considerable improvements in the health status of all child and adult patients with severe hemophilia A. In addition, it would produce five-year net savings for the Ministry of Health and the Social Security Health Insurance of US$ 58.8 million and US$ 68.7 million, respectively, considering the current number of patients. These savings and benefits are maintained by performing sensibility analysis in any model variable.
The resources saved from using emicizumab as prophylaxis for severe hemophilia A could be allocated to the prevention or treatment of other diseases.
Given the above, it is highly advisable that the public health system in Peru – including the Ministry of Health and the Social Health Insurance – implement protocols for the prophylaxis and treatment of hemophilia and fund emicizumab directly from their budget.
Notes
Contributor roles
RB: principal investigator, data collection, methodology, validation, visualization, original draft writing, revision writing, and editing. CP: literature review, data collection, methodology. PA: data collection, methodology, validation, visualization. NL, KS, CV, GC, VS: data collection, validation.
Competing interests
The authors have completed the ICMJE conflict of interest declaration form and declare receiving funding from Roche Peru for this work. The forms can be requested by contacting the corresponding author or the Editorial Direction of the Journal.
Funding
This research was financed by Roche Peru. The company did not influence the study’s design, analysis, or interpretation of the results, nor in the manuscript’s preparation, revision, or approval.
Ethics
Given the attributes of the study (open access secondary data), no ethics committee was required.
Language of submission
Spanish.

