Protocols
← vista completaPublished on August 13, 2024 | http://doi.org/10.5867/medwave.2024.07.2906
Efectos del ejercicio hipopresivo asociado con el entrenamiento aeróbico y de fuerza muscular en el tratamiento de la fatiga, los síntomas de la incontinencia urinaria, la función sexual y la calidad de vida en mujeres tratadas por cáncer ginecológico: un protocolo de ensayo clínico aleatorizado
Effects of hypopressive exercise associated with aerobic and muscle strength training on the treatment of fatigue, urinary incontinence symptoms, sexual function, and quality of life in women treated for gynecologic cancer: A randomized clinical trial protocol
Abstract
Introduction Therapeutic exercise has an important role in the population living with cancer as it improves function and quality of life and reduces the symptoms of cancer treatment. There is little clinical evidence on the effects of hypopressive exercise in women with gynecological cancer.
Objective Evaluate the effects of 4 weeks of hypopressive exercise associated with muscle strength training and aerobic exercises on fatigue, urinary incontinence symptoms, sexual function, and quality of life in women treated for gynecological cancer compared to a group that will perform conventional training.
Methods This randomized, single-blinded clinical trial study is set in the Clinical Research Laboratory, Department of Kinesiotherapy, at a Chilean University. Patients will be randomly assigned to an experimental group of hypopressive exercises associated with muscle strength training and aerobic exercises or a control group of muscle strength training and aerobic exercises. Twelve tele-rehabilitation sessions will be performed. Women over 18 years of age with gynecologic cancer who have been prescribed radiotherapy or chemotherapy will participate. Fatigue, quality of life, urinary incontinence symptoms, and sexual function will be assessed before and after the intervention.
Expected results The results of this clinical trial have important implications for specific treatment for the cancer population and generate new techniques in the practice of oncology-specialized kinesiologists. Hypopressive exercise is expected to reduce incontinence symptoms due to neuromuscular activation of the pelvic floor muscles. However, more studies are needed to confirm the beneficial effects of hypopressive exercises in face-to-face or remote rehabilitation.
Main messages
- Therapeutic exercise has an important role in the population living with cancer as it improves function and quality of life and reduces the symptoms of cancer treatment.
- There is little clinical evidence on the effects of hypopressive exercise in women with gynecological cancer.
- Knowing the effects of hypopressive exercise on pelvic floor function is relevant and could improve the results of less invasive physiotherapy interventions.
Introduction
According to the World Health Organization (WHO), cancer is the second leading cause of death worldwide; in Chile, 16% of malignancies correspond to gynecological cancer (endometrial, cervix, etc.), and during 2018 the incidence increased by 6.52%. Currently, the main oncological treatments are chemotherapy, radiotherapy, and surgery aimed at curing or stopping the progression of cancer [1]. On the other hand, cancer treatment in patients with gynecological cancer may cause secondary problems in the pelvic organs, such as pelvic floor disorders, systematic prolapse, sexual dysfunction, urinary or fecal incontinence, and reduced quality of life [2].
Rehabilitation in oncology considers different modalities of therapeutic exercises. It has been shown that exercise involving the contraction of the pelvic floor musculature is effective in reducing the symptoms of incontinence and internal organ prolapse[3]. There are several effective methods to facilitate voluntary contraction of the pelvic floor musculature: vaginal palpation, palpation of the central tendon of the perineum, interruption of urinary flow while contracting the pelvic muscles, and biofeedback using a perineometer and hypopressive exercises [4]. Most methods for strengthening the pelvic floor are invasive; however, hypopressive exercise involves principles of postural control associated with breathing andcontrolled apnea with costal opening, allowing for greater neuromuscular activation of the pelvic floor musculature [5]. Hypopressive exercise has been reported to increase pelvic floor strength in women with urinary incontinence, improving symptom control and achieving increased activation of the transverse muscle of the abdomen [6]. It also increases the cross-sectional area of the levator muscle of the anus and improves symptoms in women with pelvic organ prolapse [7]; however, there is no evidence of the effects of hypopressive exercise in adult women living with gynecological cancer.
Technological advances in the telerehabilitation modality can reduce transfer and intervention time barriers. This type of intervention has demonstrated positive effects on pain, functionality, fatigue, and quality of life in patients with advanced cancer and breast cancer survivors without reported adverse events to training [8,9]. In addition, a systematic review has shown that telerehabilitation can be an effective intervention in the care of patients living with cancer [10]. Considering the growing number of new cases of gynecological cancer in the coming years, in addition to the increase in mortality rates from this disease, it is necessary to maintain specialized care for these patients during their cancer treatment. However, there are no telerehabilitation studies considering the principles of hypopressive abdominal training associated with conventional aerobic and strength exercise training in gynecologic cancer survivors.
Objectives
The objective of this study is to evaluate the effects of hypopressive exercise associated with strength and aerobic training on fatigue, urinary incontinence symptoms, sexual function, and quality of life in women treated for gynecological cancer compared to a control group that will perform conventional training. The alternative hypothesis is that hypopressive exercise associated with conventional training is more effective in reducing fatigue and urinary incontinence symptoms, improving sexual function and quality of life in women treated for gynecological cancer compared to conventional training. The proposed study is a single-blind, randomized, parallel-group clinical trial. The allocation ratio is 1:1 and is framed as a superiority analysis.
Trial design
The proposed research design is an experimental, single-blind, randomized clinical trial. The type of design is directed to the management of two independent variables (group and training) and the single-blind designrefers to the evaluator, who will not know the participants' training type. The present study was based on the SPIRIT checklist.
Methods
Study setting
The study will take place in a home-based setting, considering the client-therapist interaction during the intervention process. At home, participants should have internet access to receive synchronous intervention from a therapist using one of the following platforms: Zoom, Google Meet, or WhatsApp. Additionally, once a week, participants should do exercises by themselves following the standardized exercise regimen provided by the therapist.
Eligibility criteria
This study will recruit 36 women aged 18 years or older (n = 18 per group). Candidates must have a confirmed diagnosis of gynecological cancer and a chemotherapy or radiotherapy indication, with or without pelvic lymphadenectomy. In addition, participants must be able to practice physical activity according to medical indications.
The exclusion criteria shall be: (a) recurrence of gynecological cancer; b) presence of lymphedema in the lower limb, measured by circumference differences between both limbs with a tape measure: asymmetries of more than 2 cm between limbs will be considered edema [11]; c) diagnosis of metastasis; (d) heart disease.
Intervention
Both exercise groups will train three times a week, twice synchronously online and once asynchronously, considering a standardized exercise regimen. In all intervention sessions, conventional exercises will be guided by the standardized exercise regimen previously developed by the study team for four weeks. Each session will be approximately 60 minutes long. In all sessions, the perceived effort (using the Borg scale) [12], respiratory rate [13], and heart rate [14] will be monitored, considering remote instructions. For aerobic training, a mild to moderate intensity (Borg 9 to 13) will be considered [15]. For respiratory and cardiac rate assessment (approximate), participants will be instructed to remain seated on a chair with a side view of their computer or phone camera so that the abdomen is visible. Then, the collaborating students will guide the participants to palpate the carotid artery with the index and the third finger on the neck, next to the trachea; once the participants claim to feel the pulsation of the artery, a student will give them a verbal command to count the number of times the pulsation is felt, the time will be recorded in 30 seconds [16] and at the same time the other collaborating students will count the respiratory rate by visualizing the diaphragmatic or apical breathing of the participants (counting the thoracic movements), during the same 30 seconds [17]. Then, the values will be multiplied by two, and the heart and respiratory rate will be recorded for one minute [17].
Muscle strength training will be performed using large muscle groups and the participant´s body weight. All exercise sessions will be the same for the two groups and will begin with a five-minute warm-up with exercises involving large muscle groups in a perceived exertion rating of 10 to 12 on the Borg scale, concluding with a 10-minute cool-down of dynamic and static muscle stretches of the main muscle groups worked on in session [17,18]. During the first week of training, familiarization with the perception of perceived effort will be carried out using the Borg scale.
Strength training will consist of three sets of 8 to 12 repetitions of eight exercises, using hip and lower limb extension, bicep flexion and extension, lunges, pectoral flexion, abdominal flexion, shoulder flexion, and back extension [18,19]. The participants will execute the exercises without any load throughout the sessions. The training will progress in volume increases as appropriate for each week: a) weeks 1 and 2 (3 sets of 8 repetitions, weeks of adaptation), b) week 3 (3 sets of 10 repetitions), and c) week 4 (3 sets of 12 repetitions).
Hypopressive exercise protocol
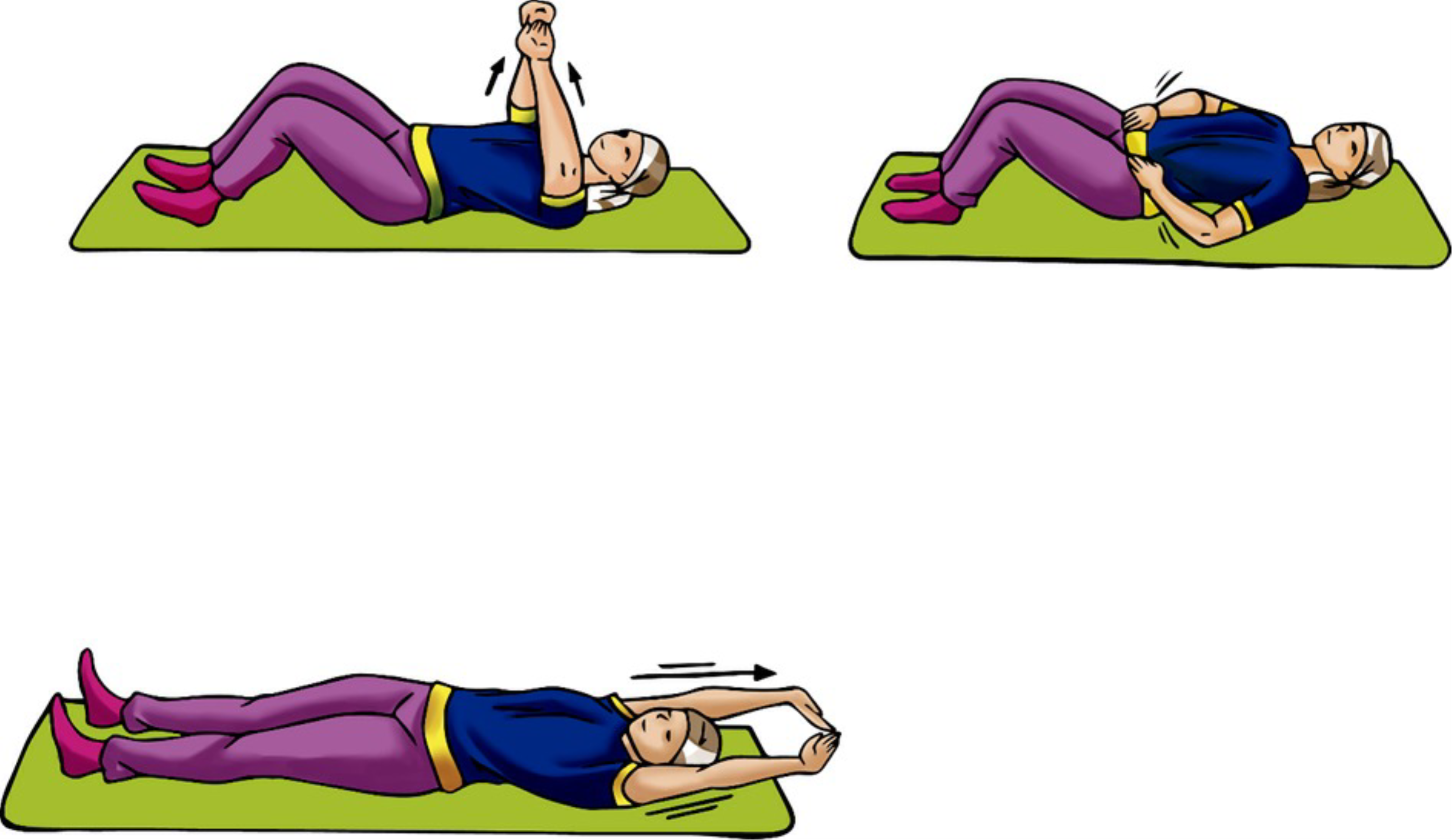
The intervention group will perform hypopressive exercises at the end of the strength and aerobic training session.. The static hypopressive exercise will be performed in a supine position, with a variant of arms in flexion, abduction, and extension, comprising three exercises. The repetitions will consist of three sets of each exercise, using a protocol adapted from the previous study of Armesilla and Andrés [20]. The participants will be placed in the lithotomy position, with extended arms to the sides of the head, both hands in flexion, and the right placed below the left. Participants must perform three cycles of deep inspiration (nose) and forced exhalation (mouth). After the last expiration, the participants will be guided to maintain the position by performing three cycles of normal breathing without performing any apnea to guarantee the safety of the user since the intervention is remote. During the expiration phase, the participants will be guided to perform pelvic floor contraction. The procedure should be repeated three times in each arm variation, as described in Figure 1, so that three breathing cycles are performed in each position. The protocol of the hypopressive exercise should last 10 minutes. Figure 1 presents the postures that will be considered during the performance of the hypopressive exercises (Figure 1 ).
Hypopressive exercises illustrations with their respective arm variations.
Primary outcome measures
Quality of life
The Quality-of-Life Questionnaire from the Quality-of-Life Group of the European Organization for Cancer Research and Treatment, Core-30 version 3.0 (EORTC QLQ-C30), will be used to assess the participants' quality of life. It consists of 30 items, and the total score varies from zero (very poor) to 100 (excellent) for functional dimensions and from zero (excellent) to 100 (very poor) for symptom dimensions [21,22]. The questionnaire has already been used in the Chilean population [23].
Sexual function
Sexual function will be evaluated using the self-report instrument Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12), validated in Spanish [24] and for adult Chilean women [25]. The questionnaire consists of 12 items, with scores ranging from 0 to 48; the higher the score, the better the sexual function [26].
Symptoms of urinary incontinence
The Pelvic Floor Impact Questionnaire-Short Form 7 (PFIQ-7) will be used to assess urinary incontinence symptoms. This instrument presents small to moderate reproducibility in women with pelvic dysfunction (effect size 0.48) [27]. The questionnaire is validated in Spanish and has high test-retest reliability[28]. Although it is not validated in the Chilean population, it was previously used in Chilean adult women [29]. This questionnaire assesses the overall impact of urinary, colorectal-anal, and genital prolapse symptoms on general activities. It is scored as follows: zero: not at all; one: somewhat; two: moderately; and three: quite a bit. To convert the score, the average score must be multiplied by 100/3, and a range of the scale from 0 to 100 is obtained; the higher the score, the greater the impact of the symptoms on general activities [27].
Fatigue
The self-report instrument Brief Fatigue Inventory (BFI), validated in Chilean Spanish, will be considered to assess the fatigue of study participants [30]. It consists of three items; the categorization of fatigue levels can be obtained through an arithmetic average of the scores obtained in each item. Zero stands for "no fatigue"; 1 to 3: "mild fatigue"; 4 to 6: "moderate fatigue" and 7 to 10: "severe fatigue" [31].
Participant´s timeline
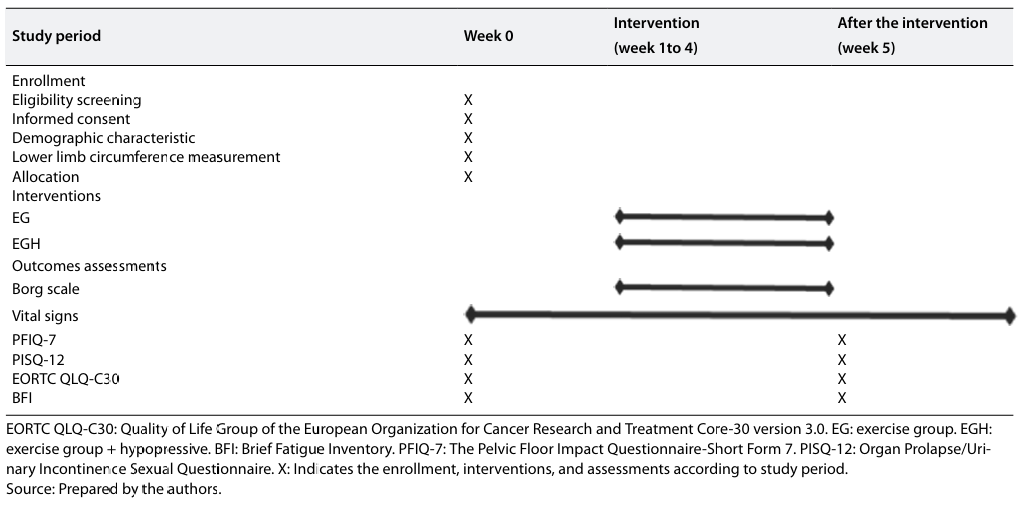
The time schedule for enrolment, allocations, interventions, and assessment is shown in Figure 2.
Time schedule for enrolment, allocations, interventions, and assessment .
Sample size
The sample size was calculated using the GPower software version 3.1, considering a Cohen´s d effect size of 0.2 to achieve a power of 80%, with a significance level of 5%. A repeated measures analysis of variance was used, considering intra- and inter-group interactions with two measurements. A total of 36 women (n = 18 per group) were suggested.
Enrollment
Patients will be referred to the study by a medical oncologist from the Regional Hospital of Talca (Maule region). The enrollment will be carried out by two trained physiotherapists, who will contact the potential study participants for an initial evaluation to determine eligibility and explain the content related to the training program and research objectives. Participants who agree to participate must sign an informed consent. Patients will be asked to refrain from participating in other types of physical training and moderate to intense physical activity during their participation in the study. Table 1 depicts the schedule of participants during the study period.
Schedule for enrollment, interventions, and assessments.
Assignment of interventions
Allocation
After selecting the sample and performing the baseline assessments, the participants will be randomly assigned to one of the two study groups. Randomization will be performed by a team member who is blind to the study protocol. A computer program will be used to perform randomization (
Implementation and blinding
The allocation, enrollment of participants, assignment to interventions, outcome assessment, and data analysis will be done by a team member blind to the study protocol. Moreover, the therapist will be blind to the assessment of participants. Participants will also be blind to the intervention group during the trial.
Data collection, management, and analysis
Data collection methods
Standardized forms will be used to record pre- and post-intervention evaluations. The registry will consider demographic data, social and health habits, and clinical variables: comorbidities, blood pressure, body mass index (weight, height), type of gynecological cancer, cancer staging, and type of cancer treatment. In addition, the dosage of the kinesic intervention will be recorded according to the Borg scale for each session.
Data management
All study information will be stored securely in the locked cabinets of the Clinical Research Laboratory, Department of Kinesiology, Universidad Católica del Maule. Each patient´s information will be classified using numerical codes and stored for five years after the completion of the research in closed filing cabinets. Access to information will be limited to people outside the research team of this study.
Statistical analysis
The statistical analysis will be performed using IBM SPSS statistical package (version 17.0). Data will be expressed in mean, standard deviation (SD), lower and upper limit of the confidence interval (CI, 95%) or median, minimum, maximum, first and third quartile, according to normality distribution using the Kolmogorov Smirnov test. To evaluate fatigue, symptoms of urinary incontinence, sexual function, and quality of life, a mixed analysis of two factors (two-way ANOVA) will be performed, considering time (pre-intervention and post-intervention) as an intra-subject and group factor (control and intervention groups) as a factor between subjects. The significance level shall be set to 0.05 for all statistical analyses. The intragroup effect sizes for the above variables will also be calculated using the Cohen d index. An effect size of more than 0.8 shall be considered large, 0.5 moderate, and less than 0.2 small [32].
Monitoring
Data monitoring will be provided by the trial committee (therapist, corresponding author). The supervision of the study, progress monitoring, and ensuring internal and external validity will also be provided by the research team (all authors associated with the trial). The assessors must inform the research team of any side effects associated with the intervention, and the research team can decide to terminate the trial at any time, including before its scheduled end, if it poses a risk to participants. Once a side effect is identified, it will be monitored to see if more participants present the same event. After that, a clinical solution will be offered, which may involve another specific intervention. If the event persists, the trial will be terminated.
Harms
Weekly, the therapist involved in the intervention must inform the research team of any side effects associated with the trial. The therapist has to ask participants if they have any problems related to the intervention. Finally, the investigator team will follow up on any events associated with the trial based on the therapist´s indications.
The research team will oversee all procedures associated with the trial to ensure compliance and conduct auditing.
Ethics and disclosure
This randomized clinical trial protocol was approved by the scientific ethics committee of the Universidad Católica del Maule, Talca, Chile (Act n°120/2021). All patients meeting the eligibility criteria will be invited to participate in this study and informed about the research procedures. Given that the evaluations will be conducted remotely, participants will have three options to choose from based on their internet access: Google Meet, Teams, or WhatsApp.
The informed consent will be sent to the participants as an attachment, and they will be asked to declare their desire to participate in this research. They will be informed about the potential side effects or risks of participating in the study. If the questions in the questionnaires cause any discomfort, the assessor will provide support and reassurance. Additionally, during the first training sessions, participants will be informed that they may experience muscle soreness due to the exercises, but this is typically does not last more than 48 hours. If the discomfort persists, the exercise will be performed at a lower intensity or will be discontinued. They will be asked to respond with one of the following options: "Yes, I agree to participate in the study" or "I do not agree to participate in the study". The intervention will be carried out with up to four online participants connected to one of the digital platforms. In WhatsApp, due to the size of the screen, only one participant will be allowed access at a time. The therapeutic exercise protocol has a low probability of overall harm and/or pain. This research protocol was recorded before the recruitment of participants at
The data collected from this study will be available only to research team members, and the dissemination of the results will not include any participants' identification data.
There are no financial nor competing interests to declare.
The study´s results will be presented at national and international conferences and published in scientific journals. Additionally, they will be shared with kinesiologists and physiotherapists who may benefit from the findings. Participants will receive an email containing a report of their individual results, as well as copies of the articles published as part of this research.
For all participants whose quality of life, sexual function, fatigue, and urinary symptoms do not improve after the study, the investigative team will offer an additional month of personalized asynchronous physiotherapy care.
The trial results will be published in an open-access journal, allowing movement-science professionals to access the final study. Additionally, partial results will be submitted as abstracts to conferences and congresses for presentation. Finally, all participants will receive a mailed report of their condition before and after the study, written in non-scientific language to facilitate understanding.

