Systematic reviews
← vista completaPublished on March 3, 2021 | http://doi.org/10.5867/medwave.2021.02.8105
Angiotensin-converting-enzyme inhibitors and angiotensin II receptor blockers for COVID-19: A living systematic review of randomized clinical trials
Inhibidores de la enzima convertidora de la angiotensina y antagonistas del receptor de angiotensina II para COVID-19: Una revisión sistemática viviente de ensayos clínicos aleatorizados
Abstract
Objective This living systematic review aims to provide a timely, rigorous, and continuously updated summary of the evidence available on the role of angiotensin-converting enzyme inhibitors (ACEi) and angiotensin II receptor blockers (ARB) in the treatment of patients with COVID-19.
Data sources We conducted searches in PubMed/Medline, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), grey literature and in a centralized repository in L·OVE (Living OVerview of Evidence), which retrieves articles from multiple sources such as PubMed/MEDLINE, Cochrane Central Register of Controlled Trials, Embase, among other pre-print and protocols repositories. In response to the COVID-19 emergency, L·OVE (Living OVerview of Evidence) was adapted to expand the range of evidence and customized to group all COVID-19 evidence in one place on a daily search basis. The search covered a period of time up to July 31, 2020.
Eligibility criteria for selecting studies and methods We adapted an already published standard protocol for multiple parallel living systematic reviews to this question's specificities. We included randomized trials evaluating the effect of either suspension or indication of angiotensin-converting-enzyme inhibitors or angiotensin II receptor blockers as monotherapy, or in combination versus placebo or no treatment in patients with COVID-19. We searched for randomized trials evaluating the effect of angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers versus placebo or no treatment in patients with COVID-19. Two reviewers independently screened each study for eligibility, extracted data, and assessed the risk of bias. We pooled the results using meta-analysis and applied the GRADE system to assess the certainty of the evidence for each outcome. We will resubmit results every time the conclusions change or whenever there are substantial updates.
Results We screened 772 records, but none was considered for eligibility. We identified 55 ongoing studies, including 41 randomized trials evaluating angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers for patients with COVID-19.
Conclusions We did not find a randomized clinical trial meeting our inclusion criteria, and hence there is no evidence for supporting the role of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers in the treatment of patients with COVID-19. A substantial number of ongoing studies would provide valuable evidence to inform researchers and decision-makers in the near future.
PROSPERO registration number CRD42020182495
Protocol preprint DOI 10.31219/osf.io/vp9nj
Main messages
- This living systematic review summarizes the available evidence available on the role of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for patients with COVID-19.
- None of the 772 records screened was eligible.
- We identified 55 ongoing studies evaluating angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers for patients with COVID-19, where 41 of them were randomized trials.
- This substantial number of ongoing studies may provide valuable evidence to inform researchers and decision-makers in the near future.
Introduction
COVID-19 is an infection caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)[1]. It was first identified in Wuhan, China, on December 31, 2019[2]; six months later, more than 16 million contagion cases have been identified across 215 countries, and more than 650 000 people have died[3]. On March 11, 2020, the World Health Organization (WHO) characterized the COVID-19 outbreak as a pandemic[1].
While most cases result in mild symptoms, some of them progress to pneumonia, acute respiratory distress syndrome, and death[4],[5],[6]. The case fatality rate reported across countries, settings, and age groups is highly variable but ranges from about 0.5% to 10%[7]. In hospitalized patients, the case fatality rate in some centers has been reported to be higher than 10%[8].
Several studies confirm that severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2)—similar to severe acute respiratory syndrome coronavirus (SARS-CoV)—uses the angiotensin‐converting enzyme 2 (ACE2) receptor for host cell entry[9],[10],[11]. So, the potential role for angiotensin-converting-enzyme inhibitors or angiotensin II receptor blockers has been the subject of much debate.
It has been theorized that patients with COVID-19 comorbid to cardiovascular diseases (such as diabetes or arterial hypertension) might present an angiotensin‐converting enzyme 2 overexpression[12]. Moreover, it has been thought that angiotensin II receptor blockers and angiotensin-converting enzyme inhibitors drugs—generally used as anti-hypertensive therapy—could cause an up-regulation effect on angiotensin‐converting enzyme 2, leading to the possibility of severe forms of COVID-19[12]. Indeed, some observational studies show both hypertension and diabetes mellitus as independent risk factors of mortality for patients with COVID-19 admitted to the hospital[13],[14]. However, the underlying mechanisms seem to be much more complex than initially thought[15]. In fact, some authors propose that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) could down-regulate the presence of angiotensin‐converting enzyme 2 receptors in lung, kidney, and heart and trigger a harmful hyperactivation of the renin-angiotensin system[10],[16],[17] just as severe acute respiratory syndrome coronavirus (SARS-CoV) has shown[18],[19].
Several comparative observational studies have been conducted attempting to elucidate the effect of angiotensin II receptor blockers or angiotensin-converting enzyme inhibitors in patients with COVID-19. Nevertheless, the lack of randomized trials and the growing amount of non-experimental studies with heterogeneous quality in reporting and methods have not facilitated a complete appraisal through systematic reviews. Indeed, up-to-date and good-quality systematic reviews are lacking. Thus, the therapeutic scope of angiotensin II receptor blockers or angiotensin-converting enzyme inhibitors in patients' clinical condition with COVID-19 remains unclear.
Using innovative and agile processes, taking advantage of technological tools, and resorting to the collective effort of several research groups, this living systematic review aims to provide a timely, rigorous, and continuously updated summary of the evidence available on the role of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers in patients with COVID-19.
Methods
Protocol and registration
This manuscript complies with the ‘Preferred Reporting Items for Systematic reviews and Meta-Analyses’ (PRISMA) guidelines for reporting systematic reviews and meta-analyses[20].
A protocol stating the shared objectives and methodology of multiple evidence syntheses (systematic reviews and overviews of systematic reviews) to be conducted in parallel for different questions relevant to COVID-19 was published elsewhere[21]. This protocol was adapted to the specificities of the question assessed in this review[22] and registered in PROSPERO (CRD42020182495).
Search strategies
Our literature search was devised by the team maintaining the L·OVE (Living OVerview of Evidence) platform, using the following approach:
- Identification of terms relevant to the population and intervention components of the search strategy, using Word2vec technology[23] to the corpus of documents available in Epistemonikos Database[24].
- Discussion of terms with content and methods experts to identify relevant, irrelevant, and missing terms.
- Creation of a sensitive Boolean strategy encompassing all the relevant terms.
- Iterative analysis of articles missed by the Boolean strategy and refinement of the strategy accordingly.
We conducted searches using L·OVE (Living OVerview of Evidence) platform for COVID-19, a system that maps PICO questions to a repository, maintained through regular searches in 27 databases, preprint servers, trial registries, and websites relevant to COVID-19. All the searches covered a period until July 31, 2020. No date or language restrictions were applied.
All the platform information comes from a repository developed and maintained by Epistemonikos Foundation through the screening of different sources relevant to COVID-19[25]. At the time of releasing this article, this repository included more than 65 000 articles relevant to the Coronavirus disease, coming from the following databases, trial registries, preprint servers and websites relevant to COVID-19: Epistemonikos database, Pubmed, EMBASE, ICTRP Search Portal, Clinicaltrials.gov, ISRCTN registry, Chinese Clinical Trial Registry, IRCT - Iranian Registry of Clinical Trials, EU Clinical Trials Register: Clinical trials for covid-19, NIPH Clinical Trials Search (Japan) - Japan Primary Registries Network (JPRN) (JapicCTI, JMACCT CTR, jRCT, UMIN CTR), UMIN-CTR - UMIN Clinical Trials Registry, JRCT - Japan Registry of Clinical Trials, JAPIC Clinical Trials Information, Clinical Research Information Service (CRiS), Republic of Korea, ANZCTR - Australian New Zealand Clinical Trials Registry, ReBec - Brazilian Clinical Trials Registry, CTRI - Clinical Trials Registry - India, DRKS - German Clinical Trials Register, LBCTR - Lebanese Clinical Trials Registry, TCTR - Thai Clinical Trials Registry, NTR - The Netherlands National Trial Register, PACTR - Pan African Clinical Trial Registry, REPEC - Peruvian Clinical Trial Registry, SLCTR - Sri Lanka Clinical Trials Registry, medRxiv Preprints, bioRxiv Preprints, SSRN Preprints, WHO COVID-19 database.
The database[24] acts as a central repository. Only articles fulfilling Epistemonikos criteria are visible to users. The remaining articles are only accessible for members of COVID-19 L·OVE (Living OVerview of Evidence) Working Group.
The searches covered from the inception date of each database until the day before submission. No study design, publication status, or language restriction were applied to the searches in Epistemonikos or the additional searches.
The following strategy was used to search in Epistemonikos Database[24]. We adapted it to the syntax of other databases.
(coronavir* OR coronovirus* OR "corona virus" OR "virus corona" OR "corono virus" OR "virus corono" OR hcov* OR "covid-19" OR covid19* OR "covid 19" OR "2019-nCoV" OR cv19* OR "cv-19" OR "cv 19" OR "n-cov" OR ncov* OR "sars-cov-2" OR "sars-cov2" OR "SARS-Coronavirus-2" OR "SARS-Coronavirus2" OR (wuhan* AND (virus OR viruses OR viral)) OR (covid* AND (virus OR viruses OR viral)) OR "sars-cov" OR "sars cov" OR "sars-coronavirus" OR "severe acute respiratory syndrome" OR "mers-cov" OR "mers cov" OR "middle east respiratory syndrome" OR "middle-east respiratory syndrome" OR "covid-19-related" OR "SARS-CoV-2-related" OR "SARS-CoV2-related" OR "2019-nCoV-related" OR "cv-19-related" OR "n-cov-related") AND (((("renin-angiotensin" OR "renin angiotensin" OR (renin* AND angiotensin*) OR "renin-angiotensin-aldosterone") AND (inhibit* OR block* OR antag* OR anti)) OR RAAS OR RAAB) OR ((("angiotensin-converting" OR (angiotensin* AND converting*) OR ACE OR "angiotensin-converting-enzyme") AND (inhibit* OR block* OR antag* OR anti)) OR aceis* OR "ace-inhibitor" OR "ace-inhibitors" OR "ace-i" OR "ace-is" OR (captopril* OR Capoten*) OR (enalapril* OR Vasotec* OR Renitec* OR Enacard*) OR (lisinopril* OR Prinivil* OR Zestril*) OR (perindopril* OR Coversyl* OR Coversum* OR Aceon*) OR (ramipril* OR Altace*) OR (quinapril* OR Accupril*) OR (benazepril* OR Lotensin*) OR cilazapril* OR (fosinopril* OR Monopril*) OR (trandolapril* OR Mavik*) OR (spirapril* OR Renormax*) OR (delapril* OR alindapril*) OR (moexipril* OR Univasc) OR temocapril* OR (zofenopril* OR Zocardis*) OR (imidapril* OR Tanatril*) OR alacepril*) OR ((("angiotensin-receptor" OR (angiotensin* AND receptor*) OR "angiotensin-ii" OR "angiotensin ii" OR "angiotensin ii-receptor") AND (inhibit* OR block* OR antag* OR anti)) OR arbs* OR "angiotensin-receptor-blocker" OR "angiotensin-receptor-blockers" OR aiira* OR (losartan* OR Cozaar*) OR (eprosartan* OR Teveten*) OR (valsartan* OR Diovan*) OR (irbesartan* OR Avapro*) OR tasosartan* OR (candesartan* OR Atacand*) OR (telmisartan* OR Micardis* OR Actavis*) OR (olmesartan* OR Benicar*) OR (azilsartan* OR Edarbi* OR Azilva* OR "TAK-536"* OR "TAK 536"* OR TAK536* OR "TAK-491"* OR "TAK 491"* OR TAK491*) OR (fimasartan* OR Kanarb*) OR abitesartan* OR elisartan* OR embusartan* OR (forasartan* OR "SC-52458" OR "SC-52458" OR SC52458*) OR milfasartan* OR saprisartan* OR zolasartan*))
Eligibility criteria
Types of studies
We planned to include randomized trials. We excluded information from non-randomized studies, post-trial analyses, and studies evaluating animal models' effects or in vitro conditions.
Types of participants
We planned to include trials assessing participants with COVID-19, as defined by the authors of the trials. Whenever we find substantial clinical heterogeneity on how the condition was defined, we planned to explore it using a sensitivity analysis.
Type of interventions
The interventions of interest were angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers. We did not restrict our criteria to any dosage, duration, timing, or route of administration. The comparison of interest was placebo (angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers plus optimal treatment versus placebo plus optimal treatment) or no treatment (angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers plus optimal treatment versus optimal treatment).
Trials evaluating angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers in combination versus placebo (angiotensin-converting enzyme inhibitors plus angiotensin II receptor blockers plus optimal treatment versus placebo plus optimal treatment) or no treatment (angiotensin-converting enzyme inhibitors plus angiotensin II receptor blockers plus optimal treatment versus optimal treatment) were eligible. Trials assessing angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers suspension were also eligible. Trials assessing angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers or angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers combination plus other drugs were eligible if the co-interventions were identical in both intervention and comparison groups.
Type of outcomes
We did not use the outcomes as an inclusion criterion during the selection process. Any article meeting all the criteria, except for the outcome criterion, was preliminarily included and assessed in full text.
We used the Core Outcome Sets for COVID-19 (COS-COVID)[26], the existing guidelines and reviews, and the authors' judgment as an input for selecting the primary and secondary outcomes, as well as to decide upon inclusion. The review team revised this list of outcomes to incorporate ongoing efforts to define Core Outcomes Sets (e.g., COVID-19 Core Outcomes)[27].
Primary outcome
● All-cause mortality
Secondary outcomes
● Mechanical ventilation
● Extracorporeal membrane oxygenation
● Length of hospital stay
● Respiratory failure
● Serious adverse events
● Time to reverse transcription-polymerase chain reaction for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 RT-PCR) negativity
Other outcomes
● Acute respiratory distress syndrome
● Total adverse events
We planned to present primary and secondary outcomes into GRADE ‘Summary of Findings’ tables[28].
Selection of studies
The results of the literature search in the Epistemonikos database were automatically incorporated into the L·OVE (Living OVerview of Evidence) platform (automated retrieval), where they were de-duplicated by an algorithm comparing unique identifiers (database ID, DOI, trial registry ID), and citation details (i.e., author names, journal, year of publication, volume, number, pages, article title, and article abstract).
Two researchers independently screened the titles and abstracts yielded by the search against the inclusion criteria. We obtained the full reports for all titles that appeared to meet the inclusion criteria or require further analysis to decide their inclusion. We recorded the reasons for excluding trials in any stage of the search and outlined the study selection process in a ‘Preferred Reporting Items for Systematic reviews and Meta-Analyses’ (PRISMA) flow diagram adapted for this project.
Extraction and management of data
Two reviewers were considered independently to extract data from each included study and would use standardized forms. We planned to collect the following information: study design, setting, participant characteristics (including disease severity and age) and study eligibility criteria; details about the administered intervention and comparison, including dose and therapeutic scheme, duration, timing (i.e., time after diagnosis) and route of administration; the outcomes assessed and the time they were measured; the source of funding of the study and the conflicts of interest disclosed by the investigators; the risk of bias assessment for each study. We planned to resolve disagreements by discussion, and one arbiter adjudicated unresolved disagreements.
Risk of bias assessment
We planned to assess the risk of bias for each randomized trial using the 'risk of bias' tool (RoB 2.0: a revised tool to assess risk of bias in randomized trials)[29]. We planned to consider the effect of assignment to the intervention for this review. Two independent reviewers were considered to assess the five domains of bias for each outcome result of all reported outcomes and time points. These five domains are, bias due to (1) the randomization process, (2) deviations from intended interventions (effects of assignment to interventions at baseline), (3) missing outcome data, (4) measurement of the outcome, and (5) selection of reported results. Answers to signaling questions and collectively supporting information were considered to lead to a domain‐level judgment in the form of 'Low risk of bias,' 'Some concerns,' or 'High risk of bias.' These domain‐level judgments were considered to inform an overall 'risk of bias' judgment for each result. Discrepancies between review authors were considered to be resolved by discussion to reach consensus. If necessary, a third review author was considered for a consultation to achieve a decision.
We planned to consider the following factors as potential baseline confounders:
● Age
● Comorbidities (e.g., cardiovascular disease, renal disease, eye disease, liver disease)
● Co-interventions
● Severity, as defined by the authors (i.e., respiratory failure vs. respiratory distress syndrome vs. intensive care unit requirement)
Measures of treatment effect
For dichotomous outcomes, we planned to express the estimate of the treatment effect of an intervention as risk ratios or odds ratios along with 95% confidence intervals. We planned to use mean difference and standard deviation to summarize the data using 95% confidence intervals for continuous outcomes. Whenever continuous outcomes are measured using different scales, we planned to express the treatment effect as a standardized mean difference with 95% confidence intervals. When possible, we planned to multiply the standardized mean difference by a standard deviation from the pooled studies, as, for example, the standard deviation from a well-known scale used by several of the studies included in the analysis on which the result is based. In cases where the minimally important difference is known, we planned to present continuous outcomes as minimally important difference units or inform the results as the difference in the proportion of patients achieving a minimal important effect between intervention and control[30]. Then, we planned to display these results on the 'Summary of Findings Table' as a mean difference[30].
Strategy for data synthesis
If we included more than one trial, we planned to conduct a formal quantitative synthesis (meta-analysis) for clinically homogeneous studies using RevMan 5[31] and using the inverse variance method with the random-effects model. For any outcomes where data were insufficient to calculate an effect estimate, we planned to present a narrative synthesis, describing the studies in terms of the direction and the size of effects, and any available measure of precision.
Subgroup and sensitivity analysis
We planned to perform subgroup analysis according to the definition of severe COVID-19 infection (i.e., respiratory failure vs. respiratory distress syndrome vs. intensive care unit requirement). In case we identified significant differences between subgroups (test for interaction < 0.05), we considered reporting the results of individual subgroups separately.
We planned to perform sensitivity analysis excluding the high risk of bias studies; and, if non-randomized studies were used, excluding studies that did not report adjusted estimates. In cases where the primary analysis effect estimates and the sensitivity analysis effect estimates significantly differ, we considered presenting either the low risk of bias-adjusted sensitivity analysis estimates or the primary analysis estimates but downgrading the evidence's certainty of risk of bias.
Assessment of certainty of the evidence
We planned to judge the certainty of the evidence for all outcomes using the Grading of Recommendations Assessment, Development and Evaluation working group methodology (GRADE Working Group)[32] across the domains of risk of bias, consistency, directness, precision and reporting bias. Certainty was considered to be adjudicated as high, moderate, low or very low. For the main comparisons and outcomes, we planned to prepare Summary of Findings (SoF) tables[28],[30] as well as interactive Summary of Findings tables. A Summary of Findings table with all the comparisons and outcomes was considered to be presented as an appendix.
Living evidence synthesis
An artificial intelligence algorithm deployed in the Coronavirus/COVID-19 topic of the platform will provide instant notification of articles with a high likelihood of being eligible. The authors will review them, decide upon inclusion, and update the review's living web version accordingly. We will consider resubmission to a journal if there is a change in the direction of the effect on the critical outcomes or a substantial modification to the certainty of the evidence.
This review is part of a larger project set up to produce multiple parallel systematic reviews relevant to COVID-19[21].
Results
Results of the search
The search in the L·OVE (Living OVerview of Evidence) platform retrieved 772 records. We considered 480 as potentially eligible and retrieved and evaluated their full texts. However, none of the studies were eligible for inclusion. That being said, 88 records were observational studies and are awaiting assessment. The reasons for exclusion - List of included studies and excluded and ongoing studies - are described in the Appendices 1 and 2.
Ongoing studies
We identified 55 ongoing studies (41 randomized trials and 14 non-randomized studies). See Appendix 1 and 2 for a list of included, excluded, and ongoing studies. The study selection process is summarized in Figure 1 - PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) Flowchart.
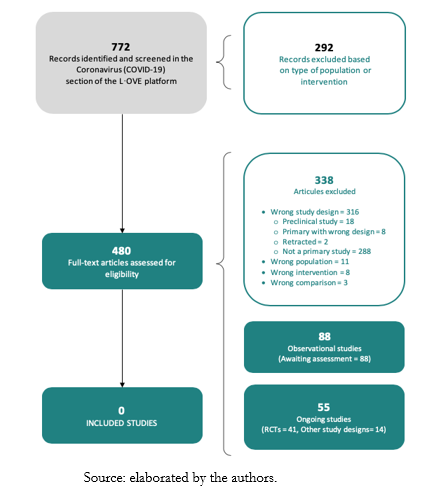 Full size
Full size Description of the studies
No study was considered eligible.
Discussion
After conducting a comprehensive search, we found no randomized trials evaluating the effect of angiotensin-converting-enzyme inhibitors or angiotensin II receptor blockers in patients with COVID-19. Given the growing body of evidence about this topic[33], we aimed to conduct a living systematic review of clinical trials and considering that our search included 41 ongoing randomized trials, future updates will be performed.
Systematic reviews are considered the gold standard for collecting and summarizing the available evidence regarding a clinical question. However, the traditional model for conducting reviews has several limitations, including high demand for time and resources[34], and rapid obsolescence[33].
In the wake of the COVID-19 crisis, researchers have made efforts to answer the urgent needs of health decision-makers during these months, although scientific rigor in some manner has been jeopardized[35]. Information is produced at a vertiginous speed[34]. Twenty-two systematic reviews have been produced—15 of them as preprints with no peer-review—aiming to provide synthesized and up-to-date evidence addressing the use of angiotensin II receptor blockers and angiotensin-converting enzyme inhibitors drugs on patients with COVID-19[36],[37],[38],[39],[40],[41],[42],[43],[44],[45],[46],[47],[48],[49],[50],[51],[52],[53],[54],[55],[56],[57]. Nevertheless, these quicker alternatives risk losing efficiency, accuracy, and rigor. Thus, only three of the 22 systematic reviews have registered a protocol[40],[41],[43], half of which were published as preprints, and six reported no assessment of risk of bias of the included studies[36],[37],[48],[53],[54],[55]. Likewise, none included randomized trials, yet none used ROBINS-I tool (ROBINS-I: Risk Of Bias In Non-randomised Studies of Interventions)[58] for assessing the risk of bias in non-randomized studies, and only one of them[52] graded the certainty of evidence using the GRADE approach[32].
Our project's main limitation is the short period of time that has elapsed since the beginning of the pandemic, which does not allow the scientific community to produce enough evidence for inclusion. Despite what our review is promising for the near future, no high-quality evidence has yet been produced to inform decision making.
Identifying, appraising, and synthesizing health research requires careful attention to a rigorous methodology, considering that systematic reviews are not updated conventionally or updated intermittently, which leaves gaps between updates. The recent missing research may put them at risk of inaccuracy[59]. Our work's main strength is that living systematic reviews address the issue of obsolescence and inaccuracy[59],[60], and help prevent waste in the contribution of new research, making them more accurate. On the other hand, integrating human-machine integration allows us to search daily and gives us high confidence about including relevant new research[60].
A living systematic review and network meta-analysis about drug treatments for COVID-19 has recently been published[61]. According to its protocol, the authors expect to include all those ongoing randomized clinical trials regarding angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers for patients with COVID-19 that meet their inclusion criteria[61].
This present review is part of a larger project set up to put such an approach into practice. This project aims to produce multiple parallel living systematic reviews relevant to COVID-19 following the higher quality standards in evidence synthesis production[17]. We believe that our methods are well suited to handle the abundance of evidence to come, including evidence on the role of angiotensin-converting-enzyme inhibitors or angiotensin II receptor blockers for COVID-19. We have identified multiple ongoing studies addressing this question, including 41 randomized trials, which will provide valuable evidence to inform researchers and decision-makers in the near future.
Conclusion
We found no randomized clinical trial meeting our inclusion criteria, and hence there is no evidence for supporting the role of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers in the treatment of patients with COVID-19. A substantial number of ongoing studies would provide valuable evidence to inform researchers and decision-makers in the near future.
During the COVID-19 pandemic, we will maintain a living, web-based, openly available version of this review, and we will re-submit the review every time the conclusions change or whenever there are substantial updates. Our systematic review aims to provide high-quality, up-to-date synthesis of the evidence useful for clinicians and other decision-makers. Up-to-date information about the group and its member organizations is available here:
Annex
Protocol preprint DOI:
10.31219/osf.io/vp9nj
Appendix 1
Appendix 1.

