Epistemonikos summaries
← vista completaPublished on December 7, 2023 | http://doi.org/10.5867/medwave.2023.11.2753
Oral atenolol compared to oral propranolol for infantile hemangioma
Atenolol oral comparado con propranolol oral para hemangioma infantil
Abstract
Introduction Infantile hemangioma is the most frequent benign vascular tumor in childhood, with an incidence of 3 to 10%. When patients require treatment, oral propranolol, a non-selective lipophilic beta-blocker, is usually considered the therapy of choice. However, its use has been associated with several adverse events related to its β-2 action and its ability to cross the blood-brain barrier. Because of this, oral atenolol, a hydrophilic β-1 receptor-selective beta-blocker, may represent a valid treatment alternative. Nonetheless, there is still controversy regarding the efficacy and safety of atenolol when compared with propranolol as monotherapy for this condition.
Methods We searched Epistemonikos, the largest database of systematic reviews in health science, which is maintained by screening multiple sources of information, including MEDLINE/PubMed, EMBASE, and Cochrane, among others. Data were extracted from the identified reviews, data from the primary studies were analyzed, a meta-analysis was performed, and a summary table of the results was prepared using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) method.
Results Nine systematic reviews were identified, including 10 primary studies and three randomized trials. The three randomized trials were included in the analysis of this investigation.
Conclusion The use of oral atenolol compared with oral propranolol as monotherapies may result in little or no difference in terms of likelihood of complete remission, decrease in Hemangioma Activity Score, likelihood of post-treatment relapse, and risk of adverse events and severe adverse events, in infantile hemangioma (low certainty of evidence).
Main messages
- There is still controversy regarding the efficacy and safety of treating infantile hemangioma with atenolol compared to propranolol as monotherapy.
- Through the FRISBEE (Friendly Summaries of Body of Evidence using Epistemonikos) and GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) methodologies, a summary and analysis comparing atenolol versus atenolol in monotherapy is presented, which allows evaluating its efficacy and safety.
- This work also presents a series of considerations that seek to guide decision-making in infantile hemangioma cases requiring treatment.
- The limitations of this work are those inherent to the applied methodologies.
Problem
Infantile hemangioma is the most common benign vascular tumor in childhood, with an estimated incidence of 3 to 10% [1,2,3].
Its clinical course is characterized by rapid growth during the first 3 to 12 months of life, to later involute spontaneously between 3 to 7 years of life [4]. Because of this, it is estimated that only 10 to 15% of patients with infantile hemangioma require some type of treatment during the proliferative phase of the disease. This treatment is usually indicated when there is ulceration, bleeding, infection, ocular or airway impairment, and/or for cosmetic reasons, such as extensive involvement of the facial region [5,6,7]. In addition to these possible complications, studies have shown that infantile hemangioma can affect the mental health of both children and their families [8,9].
Among the available treatment options, propranolol, a non-selective beta-blocker, is usually considered the first-line therapy [2,9,10]. However, its use may be associated with various adverse events, mainly related to its β-2 action and its ability to cross the blood-brain barrier due to its lipophilic nature [6,11,12]. Among these adverse events, bronchial obstruction, hypotension, hypoglycemia, seizures, sleep disturbances, and gastrointestinal symptoms, among others, have been described [5,6,11,12].
On the other hand, atenolol, a β-1 receptor selective beta-blocker, could represent a valid treatment alternative, potentially avoiding the adverse events described with the use of propranolol due to its lack of β-2 activity and its hydrophilic nature, which prevents it from crossing the blood-brain barrier [11].
Other therapeutic alternatives that have been studied are the topical use of timolol and/or imiquimod; intralesional or oral corticosteroid therapy; chemotherapeutic drugs such as vincristine and interferon; use of different laser modalities; and surgery [5,10].
This review aims to evaluate the efficacy and safety of using oral atenolol in patients with infantile hemangioma, compared to oral propranolol as monotherapy.
Methods
We searched Epistemonikos, the largest database of systematic reviews in health sciences, which is maintained by searching multiple sources of information, including MEDLINE/PubMed, EMBASE, and Cochrane, among others. Data were extracted from the identified reviews, and data from the primary studies were analyzed. With this information, a structured summary called FRISBEE (Friendly Summaries of Body of Evidence using Epistemonikos) was generated, following a pre-established format, including key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the studies when possible, a summary table of results with the GRADE (Grading of Recommendations, Assessment, Development and Evaluation) method, and a section of further considerations for decision-making.
Summary of results.
Information on the effects of oral atenolol compared with oral propranolol is based on three trials [5,11,17] involving 440 patients. All trials measured complete remission following the completion of treatment. Two trials [11,17] evaluated the decrease in Hemangioma Activity Score, and two trials [5,11] measured post-treatment relapse. All trials [5,11,17] analyzed the presence of adverse events and severe adverse events in both groups.
The summary of the results is as follows:
- The use of atenolol compared with the use of propranolol as monotherapy may result in little or no difference in the likelihood of complete remission of infantile hemangioma (low certainty of evidence).
- The use of atenolol compared with the use of propranolol as monotherapy could have little or no difference in terms of decreasing the Hemangioma Activity Score in infantile hemangioma (low certainty of evidence).
- The use of atenolol compared with the use of propranolol as monotherapy could result in little or no difference in the likelihood of post-treatment relapse in infantile hemangioma (low certainty of evidence).
- The use of atenolol compared with the use of propranolol as monotherapy could result in little or no difference in the risk of adverse events and severe adverse events (low certainty of evidence).
How we conducted this summary
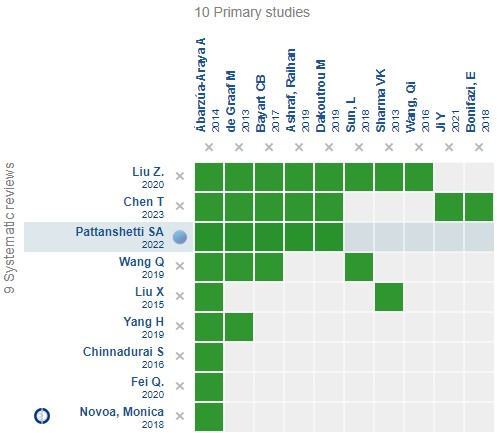
Using automated and collaborative methods, we compiled all the evidence relevant to the question of interest and presented it in an evidence matrix (Figure 1).
Evidence matrix for the question of interest.
 Full size
Full size An evidence matrix is a table that compares systematic reviews that answer the same question.
The rows represent the systematic reviews, and the columns show the primary studies.
The green boxes correspond to studies included in the respective reviews.
The system automatically detects new systematic reviews, including any primary studies in the matrix, which will be added if they answer the same question.
Follow the link to access the interactive version:
Notes
If new systematic reviews on this topic are published after this abstract is published, a "new evidence" notice will be displayed at the top of the matrix. While the project envisages regular updating of these abstracts, users are invited to comment on the Medwave website or contact the authors by e-mail if they believe there is evidence that warrants an earlier update.
After creating an Epistemonikos account, by saving the matrices, you will receive automatic notifications whenever there is new evidence that potentially answers this question.
This article is part of the Epistemonikos evidence synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and an internal peer-review process. Each of these articles corresponds to a summary called FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question in a friendly format for clinicians. Its main resources are based on the Epistemonikos evidence matrix and analysis of results using GRADE methodology. Further details of the methods to elaborate this FRISBEE are described here (
The Epistemonikos Foundation is an organization that seeks to bring information closer to health decision-makers through the use of technologies. Its main development is the Epistemonikos database (

