Epistemonikos summaries
← vista completaPublished on December 8, 2015 | http://doi.org/10.5867/medwave.2015.6330
Is tranexamic acid effective for acute upper gastrointestinal bleeding?
¿Es efectivo el ácido tranexámico en la hemorragia digestiva alta aguda?
Abstract
Upper gastrointestinal bleeding constitutes a medical-surgical emergency given its important associated morbidity and mortality. The antifibrinolytic tranexamic acid might help stopping bleeding, but controversy remains about its role in this setting. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified five systematic reviews including eight randomized trials. We combined the evidence using meta-analysis and generated a summary of findings table following the GRADE approach. We concluded tranexamic acid probably decreases rebleeding and mortality, without increasing thromboembolic adverse effects in patients with upper gastrointestinal bleeding.
Problem
Acute upper gastrointestinal bleeding is a common condition in emergency departments and critical care units. Most cases are self-limited, but there is a percentage of patients close to 30% in which bleeding persists or recurs, leading to high morbidity and mortality [1].
Tranexamic acid, an antifibrinolytic that reduces fibrin degradation assisting in the formation of blood clot, has been postulated among treatment options. This drug has proven effectiveness in trauma patients, but its role in upper gastrointestinal bleeding is not yet clearly defined.
Methods
We used Epistemonikos database, which is maintained by screening more than 30 databases, to identify systematic reviews and their included primary studies. With this information, we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found five systematic reviews [2],[3],[4],[5],[6] that consider 11 primary studies reported in 13 references [7],[8],[9],[10],[11],[12],[13],[14],[15],[16],[17],[18],[19], including eight randomized controlled trials [7],[8],[9],[10],[11],[13],[14],[19]. This table and the summary in general are based on the latter. |
|
What types of patients were included |
In six studies, the average age exceeded 50 years [7],[8],[9],[13],[14],[19]. The remaining two studies did not report the age of the patients [10],[11] Confirmation of gastrointestinal bleeding was performed using medical records in one study [14], through the presence of hematemesis or melena in four studies [8],[9],[10],[11], with upper gastrointestinal endoscopy in two studies [7],[19], and one study did not report this aspect [13]. Regarding severity, two studies included patients with massive gastrointestinal bleeding and unstable hemodynamics [9],[13], one study included severe upper gastrointestinal bleeding [7], and the remaining five studies did not report severity [8],[10],[11],[14],[19]. |
|
What types of interventions were included |
Three studies administered tranexamic acid orally [9],[11],[14], while the other five studies used it orally or intravenously. One study used tranexamic acid for less than three days [9], two studies between 3 and 4 days [7],[14] and five studies for over 5 days to a maximum of 7 days [8],[10],[11],[13],[19]. All studies compared against placebo or standard treatment. |
|
What types of outcomes |
Different systematic reviews reported meta-analysis for the following outcomes: Mortality, rebleeding, need for surgery, required transfusions, adverse events (acute myocardial infarction, pulmonary embolism, stroke, deep vein thrombosis). |
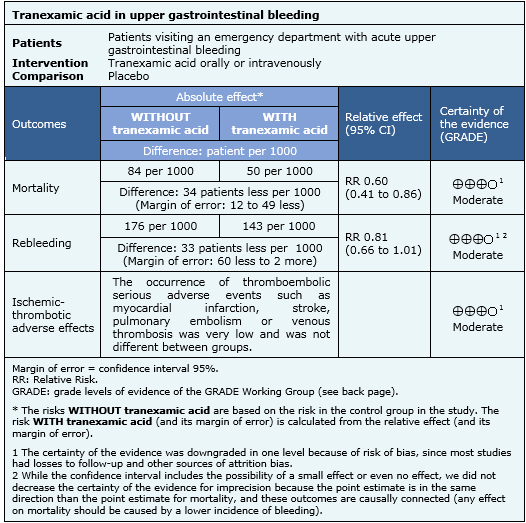
Summary of findings
Information on the effects of tranexamic acid in upper gastrointestinal bleeding is based on eight randomized controlled studies involving 1701 patients [7],[8],[9],[10],[11],[12],[13],[14],[19]. All studies provided information on mortality, and seven studies provided information on risk of rebleeding [7],[8],[10],[11],[13],[14],[19].
- Tranexamic acid probably reduces mortality in upper gastrointestinal bleeding. The certainty of the evidence is moderate.
- Tranexamic acid probably reduces rebleeding in upper gastrointestinal bleeding. The certainty of the evidence is moderate.
- Tranexamic acid probably does not increase the risk of thromboembolic serious adverse effects. The certainty of the evidence is moderate.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
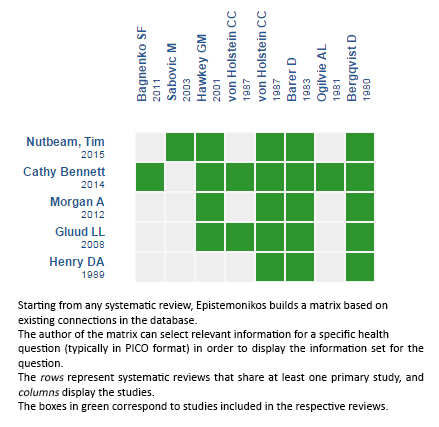
Follow the link to access the interactive version: Tranexamic acid for upper gastrointestinal bleeding
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

