Epistemonikos summaries
← vista completaPublished on December 25, 2015 | http://doi.org/10.5867/medwave.2015.6346
What are the effects of omalizumab in refractory chronic spontaneous urticaria?
¿Cuáles son los efectos del omalizumab en pacientes con urticaria crónica espontánea refractaria?
Abstract
Chronic spontaneous urticaria is a disorder mediated by mast cells, characterized by the development of wheals, angioedema or both, lasting six weeks or more, with or without a known trigger agent. First and second line treatment are antihistamines, but some refractory cases require other alternatives, such as omalizumab. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified four systematic reviews including five pertinent randomized controlled trials overall. We combined the evidence using meta-analysis and generated a summary of findings following the GRADE approach. We concluded omalizumab reduces symptoms and improves quality of life in patients with chronic spontaneous urticaria.
Problem
Chronic spontaneous urticaria is a disorder mediated by mast cells, characterized by wheals, angioedema or both, lasting six weeks or more. Usual treatment is based on H1 antihistamines, but some patients do not achieve an optimal clinical response even with maximal doses. Different alternatives have emerged for second and third line treatment of this condition, such as omalizumab, a monoclonal antibody that selectively binds to IgE. It has been postulated that omalizumab would improve symptoms and quality of life. The main potential adverse effects are headache, abdominal pain and injection site reaction.
Methods
We used Epistemonikos database, which is maintained by screening more than 30 databases, to identify systematic reviews and their included primary studies. With this information, we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found four systematic reviews [1],[2],[3],[4] that include five primary studies [5],[6],[7],[8],[9], all of which correspond to randomized controlled trials. |
|
What types of patients were included |
Five studies included adults with chronic spontaneous urticaria defined by increased itching or hives lasting for 6-8 weeks despite treatment with antihistamines. Three studies included patients with UAS7 (Urticaria Activity Score) > 16 points [5],[6],[7], one study with UAS > 4 points [8] and one study with UAS7 > 10 points [9]. |
|
What types of interventions were included |
The intervention was omalizumab versus placebo in all the studies, maintaining the antihistamine baseline treatment. One study used omalizumab 300 mg/day [5], one study used 75-375 mg/day [9], two studies used doses of 75, 150 and 300 mg/day [6],[7], and one study used doses of 75, 300 and 600 mg/day [8]. The treatment period with omalizumab lasted 24 weeks in three studies [5],[6],[9], 12 weeks in one study [7] and 4 weeks in one study [8]. |
|
What types of outcomes |
The outcomes measured were the change in the index of disease and quality of life. Index of disease was measured with UAS7, which evaluates itching and hives for seven days with a scale of 0-6 points per day, with a minimum of 0 points and a maximum of 42 points. Quality of life was evaluated with CU-Q2oL (Chronic Urticaria Quality of Life Questionnaire) which evaluates 23 factors ranging from 0 to 5 points, with a minimum of 0 points and a maximum of 115. |
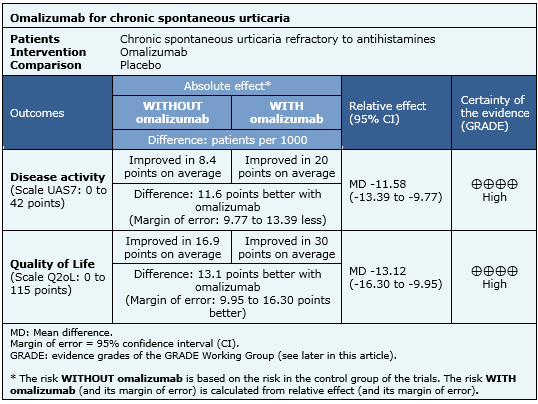
Summary of findings
The information on the effects of omalizumab is based on five randomized studies including 1117 patients. All studies measured improvement with UAS7 scale and four studies measured improvement with Q2oL [5],[6],[7],[8],[9].
- Omalizumab decreases disease activity in patients with chronic spontaneous urticaria refractory to antihistamines. The certainty of the evidence is high.
- Omalizumab improves quality of life of patients with chronic spontaneous urticaria refractory to antihistamines. The certainty of the evidence is high.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
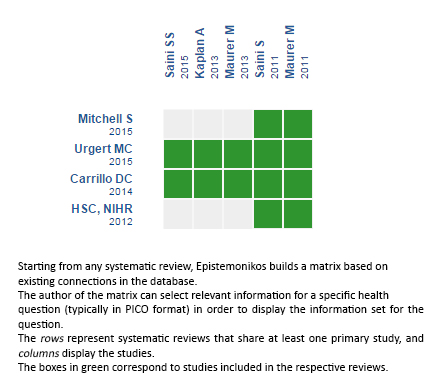
Follow the link to access the interactive version: Omalizumab versus placebo for chronic spontaneous urticaria
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

