Epistemonikos summaries
← vista completaPublished on January 9, 2016 | http://doi.org/10.5867/medwave.2015.6361
Is ursodeoxycholic acid effective for intrahepatic cholestasis of pregnancy?
¿Es efectivo el ácido ursodeoxicólico en la colestasia intrahepática del embarazo?
Abstract
Intrahepatic cholestasis of pregnancy is a condition associated with fetal morbidity and mortality. Ursodeoxycholic acid has been proposed as a treatment alternative, but its use remains controversial. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified three systematic reviews including eight randomized trials. We combined the evidence using meta-analysis and generated a summary of findings table following the GRADE approach. We concluded ursodeoxycholic acid reduces prematurity risk and need for admission in neonatal intensive care units. It might also reduce maternal pruritus.
Problem
Intrahepatic obstetric cholestasis is an exclusive condition of pregnancy. The highest prevalence is in South America and Scandinavia, where it is about 6-29% [1]. It is characterized by the appearance of severe palmoplantar pruritus predominantly nocturnal, during the third trimester of pregnancy, which may be associated with elevated liver enzymes and bile acids. The etiology of this condition is not completely understood, but genetic, hormonal and environmental factors would be involved [2]]. It has been associated to an increase in premature delivery, neonatal respiratory distress syndrome and spontaneous fetal death, among others.
Ursodeoxycholic acid could reduce symptoms and adverse outcomes decreasing hepatocellular damage and placental transfer of bile acids by replacing toxic endogenous bile acids without altering the biliary function [3],[4]. However, there is controversy about the real usefulness of this intervention.
Methods
We used Epistemonikos database, which is maintained by screening more than 30 databases, to identify systematic reviews and their included primary studies. With this information, we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found three systematic reviews [5],[6],[7], including 14 studies reported in 21 references [8],[9],[10],[11], |
|
What types of patients were included |
All of the studies included pregnant women with clinical diagnosis of obstetric cholestasis. Six studies reported when pruritus started; in four studies started at week 29 [11],[12],[22],[23] and in two at week 24 [10],[17]. Five studies also used laboratory test results (bile acids > 10 and/or aminotransferase increase) as inclusion criteria [10],[11],[12],[17],[23]. Two studies did not describe inclusion criteria [14],[16]. Age and race were not considered as inclusion criteria in any study. |
|
What types of interventions were included |
All of the studies used oral ursodeoxycholic acid. Daily dose was different across studies: 600 mg [11],[22], 900 mg [16],[17], 1000 mg [12],[23], 500-2000 mg [10] and it was not specified in one study [14]. The intervention was administered during 14 days in two studies [16],[17], 20 or more days in three studies [11],[12],[22] and it was not specified in three studies [10],[14],[23]. All of the studies compared against placebo. |
|
What types of outcomes |
Several outcomes were measured in the studies. The outcomes were divided in maternal outcomes (reduction of pruritus, aminotransferase and bile acid levels, cesarean section and adverse effects) and neonatal outcomes (prematurity, stillbirth, admission to neonatal intensive care unit, meconium staining of amniotic fluid, birth weight and fetal distress). |
|
We found three systematic reviews [5], [6], [7], including 14 studies reported in 21 references [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28]. Eight studies correspond to randomized controlled trials (15 references [9], [10], [11], [12], [13], [14], [16], [17], [19], [21], [22], [23], [24], [26], [27]). This table and the summary in general are based on the latter. One study [15] did not contribute data to any of the outcomes of interest. |
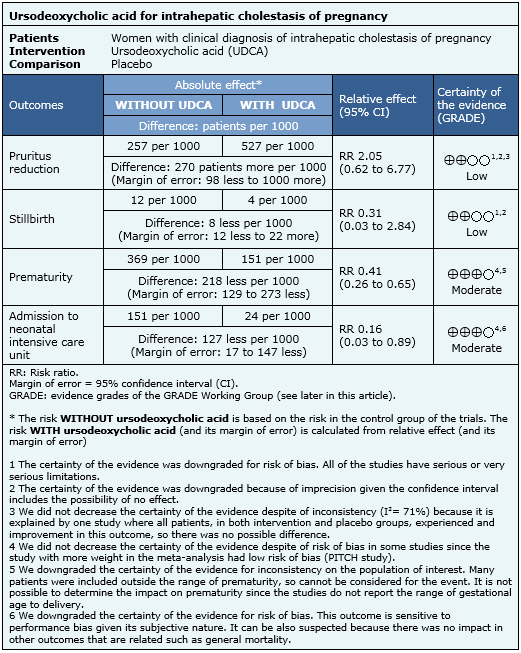
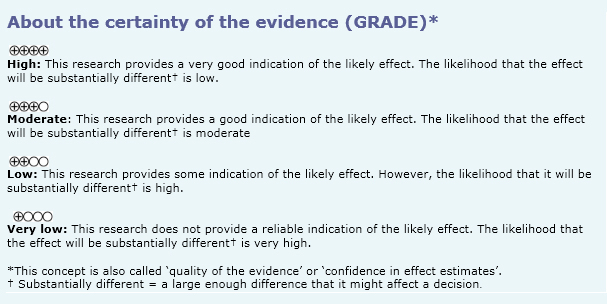
Summary of findings
The information on the effects of ursodeoxycholic acid in intrahepatic cholestasis of pregnancy is based on eight randomized controlled trials that included 363 patients. Four studies reported pruritus improvement [11],[12],[22],[23], six studies stillbirth [10],[11],[12],[14],[17],[23], six studies prematurity [10],[11],[12],[17],[22],[23] and four studies reported admission to neonatal intensive care unit [10],[11],[22],[23].
- Ursodeoxycholic acid might reduce pruritus in women with intrahepatic cholestasis of pregnancy. The certainty of the evidence is low.
- Ursodeoxycholic acid might reduce stillbirth risk in women with intrahepatic cholestasis of pregnancy. The certainty of the evidence is low.
- Ursodeoxycholic acid probably reduces prematurity risk in intrahepatic cholestasis of pregnancy. The certainty of the evidence is moderate.
- Ursodeoxycholic acid probably reduces admission to neonatal intensive care unit in intrahepatic cholestasis of pregnancy. The certainty of the evidence is moderate.
Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
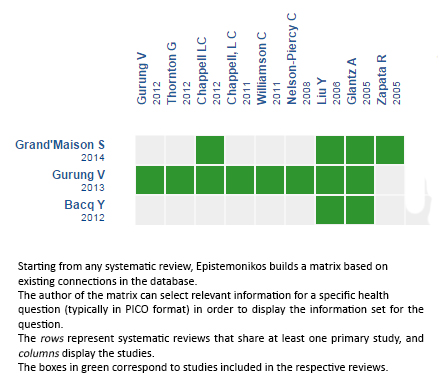
Follow the link to access the interactive version: Ursodeoxycholic acid versus placebo for intrahepatic cholestasis of pregnancy
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

