Epistemonikos summaries
← vista completaPublished on September 14, 2016 | http://doi.org/10.5867/medwave.2016.6539
Are cannabinoids effective for treatment of pain in patients with active cancer?
¿Son efectivos los cannabinoides para el tratamiento del dolor en pacientes con cáncer activo?
Abstract
Cannabinoids have been proposed for the treatment of patients with cancer pain, especially if standard treatment does not control symptoms. Using Epistemonikos database, which is maintained by searching 30 databases, we identified nine systematic reviews including seven trials that answer the question of interest, of which six are randomized trials. We performed a meta-analysis and generated a summary of findings table using the GRADE approach. We concluded it is unclear whether cannabinoids decrease pain and improve quality of life in patients with refractory cancer pain because the certainty of the evidence is very low, and it probably increases adverse effects substantially.
Problem
Cannabis has been used for centuries both recreationally and therapeutically. However, its use has been restricted since the United Nations banned it in 1971. The interest in potential therapeutic uses led to the discovery of endocannabinoid receptors CB1 and CB2, which through a G-protein associated mechanism, would lead to reduced pain. Considering many cancer patients persist with pain despite maximal analgesic therapy, plant extracts with the active agent delta-9-tetrahydrocannabinol or its analogues have been proposed. However, it is unclear what their real clinical role is.
Methods
We used Epistemonikos database, which is maintained by screening more than 30 databases, to identify systematic reviews and their included primary studies. With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We identified nine systematic reviews [1],[2],[3],[4],[5], [6],[7],[8],[9], including seven primary studies [10],[11], [12],[13],[14],[15],[16] of which six are randomized controlled trials [10],[11],[12],[13],[14],[15]. This table and the summary in general are based on the latter. |
|
What types of patients were included |
All of the trials included adults with active cancer and pain of diverse etiology (cancer, bone or neuropathic) [10],[11],[12],[13],[14],[15]. Four trials included inpatients [10],[11],[12],[13], and two outpatients [14],[15]. Only two trials [14],[15] included patients that were refractory to treatment, defined as moderate or intense pain despite treatment with 80 mg or more morphine equivalents. |
|
What types of interventions were included |
Three trials evaluated delta-9-tetrahydrocannabinol extracts [10],[11],[14], two trials [14],[15] addressed nabiximol, a preparation of tetrahydrocannabinol and cannabidiol, one trial tested a nitrogen analogue of Cannabis (NIB) [12] and one trial evaluated benzopyranoperidine [13]. All of the studies compared against placebo or treatment with codeine (in less than standard dose). One trial had several arms, including comparison against other cannabinoids [14]. |
|
What types of outcomes |
The main outcome pooled by the systematic reviews identified was pain reduction evaluated with Houde’s scale, visual-analogue scale or numerical-rating scale. Other outcomes pooled were quality of life, and adverse effects such as gastrointestinal (nausea, vomiting), central nervous system (ataxia, memory impairment, disorientation, confusion), psychiatric (euphoria, depression, anxiety, psychosis), and death, among others. |
Summary of findings
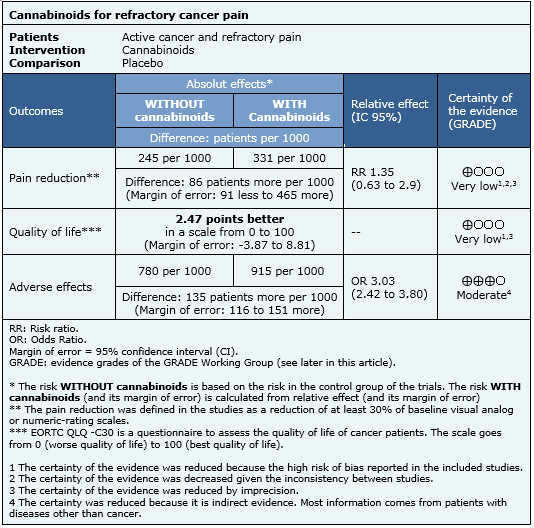
The information on the effects of cannabinoids is based on two randomized trials (290 participants) that evaluated patients with refractory pain [14],[15]. Both trials measured reduction of pain and one reported quality of life [14]. The information on adverse effects is based on a systematic review [7] evaluating adverse effects of cannabinoids in different populations, and includes 29 studies reporting this outcome.
- It is unclear whether cannabinoids reduce pain in patients with refractory cancer pain, because the certainty of the available evidence is very low.
- It is unclear whether cannabinoids improve quality of life in patients with refractory cancer pain, because the certainty of the available evidence is very low.
- Cannabinoids probably lead to a substantial increase on adverse events in patients with active cancer and refractory pain. The certainty of the evidence is moderate.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
| Feasibility and acceptability |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
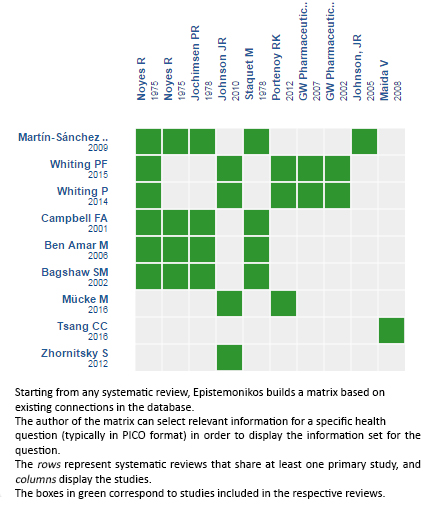
Follow the link to access the interactive version: Cannabinoids for treatment of pain in patients with active cancer
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

