Epistemonikos summaries
← vista completaPublished on November 11, 2016 | http://doi.org/10.5867/medwave.2016.6599
Are intraarticular steroids effective for knee osteoarthritis?
¿Son efectivos los corticoides intraarticulares en artrosis de rodilla?
Abstract
Knee osteoarthritis is a chronic disabling condition that is both progressive and irreversible. Intraarticular steroids are commonly used to reduce osteoarthritis symptoms and to minimize the need for surgery. Nevertheless, debate still exists regarding the efficacy and safety of steroids. To address this point, we searched Epistemonikos database which is maintained by screening 30 separate databases and identified 12 systematic reviews including 41 studies addressing steroids use in knee osteoarthritis. Of these, 40 were randomized trials. The evidence from these studies was combined using meta-analysis, and a summary of findings table was constructed following the GRADE approach. We concluded intraarticular steroid use slightly decreases short-term pain, makes little or no difference in the mid-term, and may have no effects in the long-term.
Problem
Knee osteoarthritis is one of the main causes of knee pain in the general population, affecting over 250 million people globally [1]. Some studies show that up to 10% of patients over 55 have some degree of disabling osteoarthritis, and one quarter of these patients could be severely disabled [2]. This is particularly relevant when considering the chronic and irreversible evolution of this disease, which progresses 4% annually and presents a symptomatic conversion of 1% per year [3]. Treatment for this disease is focused on pain management and maintaining patient functionality. Since knee osteoarthritis originates from degenerative and inflammatory mechanisms [4], it is widely held that intraarticular steroid administration would decrease the inflammatory response associated with this disease.
Methods
We used Epistemonikos database, which is maintained by screening more than 30 databases, to identify systematic reviews and their included primary studies. With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found 12 systematic reviews [5],[6],[7],[8],[9],[10], [11],[12],[13],[14],[15],[16], which included 41 primary studies [17],[18],[19],[20],[21],[22],[23],[24],[25],[26], [27],[28],[29],[30],[31],[32],[33],[34],[35],[36],[37], [38],[39],[40],[41],[42],[43],[44],[45],[46],[47],[48], [49],[50],[51],[52],[53],[54],[55],[56],[57]. All of these primary studies [42], except one, were randomized controlled trials. Of the primary studies, 14 randomized trials specifically addressed the topic of the present report. These 14 trials were used to create this summary [17],[18],[19],[20], [21],[22],[23],[24],[25],[26],[27],[28],[29],[30]. |
|
What types of patients were included |
All of the studies included patients diagnosed with symptomatic knee osteoarthritis and with symptoms (i.e. pain) lasting more than six months. On average, patients had between 55 and 70 years-old, were mostly women (61-93%), and included patients with grade II-IV Kellgren-Lawrence osteoarthritis. |
|
What types of interventions were included |
Three studies used prednisolone acetate [24],[25],[38], seven used triamcinolone hexacetonide [17],[18],[19],[20],[23],[26],[30], three used hydrocortisone [20],[21], [27], two used methylprednisolone [18], [21], one used betamethasone [18], and one used cortivazol [22]. |
|
What types of outcomes |
The evaluated outcomes were pain within the first 2 weeks, 4-6 weeks, 3 months, and 6 months. Additionally, functionality was evaluated using the Western Ontario and McMaster Universities Arthritis Index (WOMAC). The different studies evaluated WOMAC outcomes between 9 and 34 weeks post-treatment. Other evaluated outcomes included severe complications and treatment discontinuation due to adverse effects. |
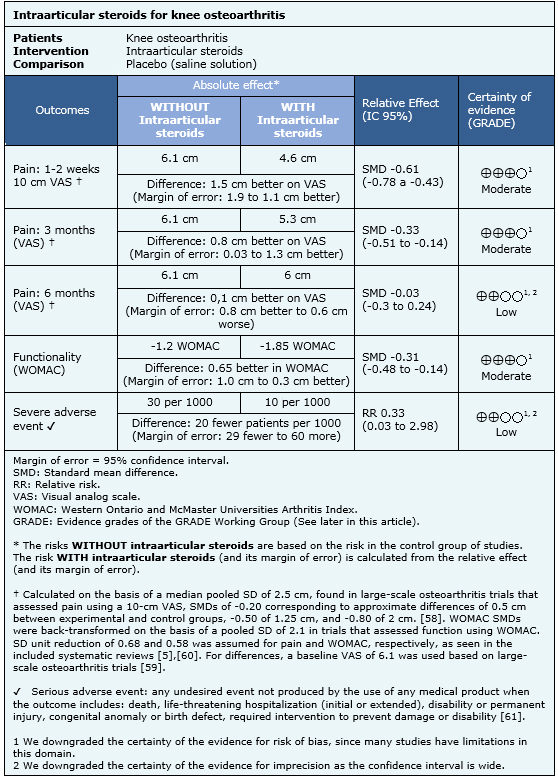
Summary of findings
The effects of intraarticular steroids were determined based on 14 randomized, controlled trials that, in total, included 810 patients [17],[18],[19],[20],[21],[22],[23],[24],[25],[26],[27],[28],[29],[30]. Nine trials (591 patients) measured pain after 1 or 2 weeks [17],[18],[19],[20],[22],[25],[26],[28],[30]; eight trials (539 patients) measured pain after 3 months [17],[18],[22],[23],[25],[28],[29],[30]; three trials (271 patients) measured pain after 6 months [22],[27],[28]; eight trials (568 patients) reported functionality [18],[19],[20], [22],[23],[28],[29],[30]; and three trials (218 patients) reported severe adverse events [22],[28],[29].
The findings can be summarized as follows:
- Intraarticular steroid injection probably lead to a slight, short-term reduction in pain. The certainty of this evidence is moderate.
- Intraarticular steroid injection probably insignificantly decrease pain in the mid-term. The certainty of this evidence is moderate.
- Intraarticular steroid injection might not make any difference in long-term pain. The certainty of this evidence is low.
- Intraarticular steroid injection probably insignificantly improve functionality. The certainty of this evidence is moderate.
- Intraarticular steroid injection might have no relevant complications. The certainty of this evidence is low.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
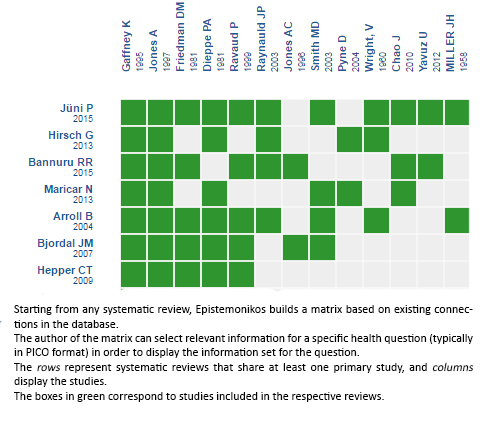
Follow the link to access the interactive version: Intraarticular corticosteroids for knee osteoarthritis
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

