Epistemonikos summaries
← vista completaPublished on December 5, 2016 | http://doi.org/10.5867/medwave.2016.6632
Does the addition of ezetimibe to statins reduce cardiovascular risk?
¿Disminuye el riesgo cardiovascular al agregar ezetimibe al tratamiento con estatinas?
Abstract
Statins are the mainstay of lipid-lowering therapy nowadays, since they reduce cardiovascular risk when used as primary or secondary prevention. However, only one third of the patients reach the goals established in several guidelines, and even if they do, they keep a risk higher than healthy controls. One of the new lipid-lowering agents is ezetimibe. Searching in Epistemonikos database, which is maintained by screening multiple databases, we identified nine systematic reviews comprising 67 trials overall. We combined the evidence using meta-analysis and generated a summary of findings following the GRADE approach. We concluded adding ezetimibe to statins probably results in little or no difference in overall mortality. It might lead to a small reduction in the risk of myocardial infarction and stroke, but the certainty of the evidence is low.
Problem
Cardiovascular disease is the leading cause of death in adults. Higher LDL cholesterol levels are associated to an increase in the risk of coronary artery disease. This risk decreases with lower LDL levels until approximately 80 mg/dl (2.1 mmol/l) [1]. Therefore, multiple clinical guidelines have set LDL goals, which in many cases require intensive treatment [1]. Even though high dose statin regimen constitutes an option, many patients do not achieve the goals. In addition, even when they do, their cardiovascular risk remains higher than the general population, an effect called residual risk. New alternatives have emerged, such as ezetimibe, a cholesterol absorption inhibitor that blocks the intestinal absorption of dietary and biliary cholesterol and related plant sterols without affecting the uptake of triglycerides or fat-soluble vitamins. Considering its mechanism differs from statins, which reduce cholesterol synthesis, they might be a good choice for combination treatment. However, it remains unclear if adding ezetimibe to statin treatment has benefits, especially in reducing long-term cardiovascular risk.
Methods
We used Epistemonikos database, which is maintained by screening multiple databases, to identify systematic reviews and their included primary studies. With this information, we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found nine systematic reviews [2], [3], [4], [5], |
|
What types of patients were included |
Nine trials included patients with coronary artery disease [26], [27], [37], [42], [48], [53], [54], [71], [74]; two of them included participants with LDL levels below 160 mg/dl [42],[71]. One trial included patients with hypercholesterolemia and high cardiovascular risk [58], one trial was conducted on patients with acute coronary syndrome [29], one trial on patients with acute myocardial infarction [69], one trial included diabetic patients with hypercholesterolemia [17], two trials included patients with peripheral artery disease [50], [76]; two trials included patients with chronic kidney disease [31], [73] and 18 trials included patients with primary hypercholesterolemia [12], [18], [19], [23], [24], [28], [34], [35], [36], [40], [43], [46], [65], [66], [67], [68], [77], [78]. One trial included African-American participants only [18]. One trial included homozygous familial hypercholesterolemia [52]; and one trial was conducted on healthy subjects [49]. |
|
What types of interventions were included |
Five trials compared the effects of atorvastatin and ezetimibe versus atorvastatin plus placebo [26], [42], [66], [67], [74]; 22 trials simvastatin plus ezetimibe versus simvastatin plus placebo [17], [18], [19], [23], [28], [29], [31], [35], [37], [40], [43], [46], [49], [53], [54], [68], [69], [71], [73], [76], [77], [78]; one trial atorvastatin and simvastatin associated with ezetimibe versus both statins plus placebo separately [52]; one trial simvastatin plus ezetimibe versus atorvastatin plus placebo [65]; six trials compared pravastatin, lovastatin, fluvastatin or several statins in the same trial associated with ezetimibe versus statin monotherapy [12], [24], [34], [36], [48], [58]; and two trials compared rosuvastatin plus ezetimibe versus rosuvastatin plus placebo [27], [50]. |
|
What types of outcomes |
Even though the primary studies reported outcomes in different ways, the systematic reviews identified grouped them as follows:
|
Summary of findings
The information about the effects of ezetimibe associated to statins is based on 37 randomized controlled trials [12], [17], [18], [19], [23], [24], [26], [27], [28], [29], [31], [34], [35], [36], [37], [40], [42], [43], [46], [48], [49], [50], [52], [53], [54], [58], [65], [66],[67], [68], [69], [71], [73], [74], [76], [77], [78]. Only nine trials reported overall mortality [29], [31], [42], [43], [50], [66], [73], [74], [76], seven reported nonfatal myocardial infarction [27], [29], [43], [50], [73], [74], [76], six informed nonfatal stroke [31], [43], [50], [73], [74], [76], three reported coronary revascularization [27], [29], [74] and 17 analyzed serious adverse events [17], [23], [24], [27], [29], [30], [31], [37], [42], [46], [53], [54], [64], [66], [71], [73], [78]. The summary of findings is the following:
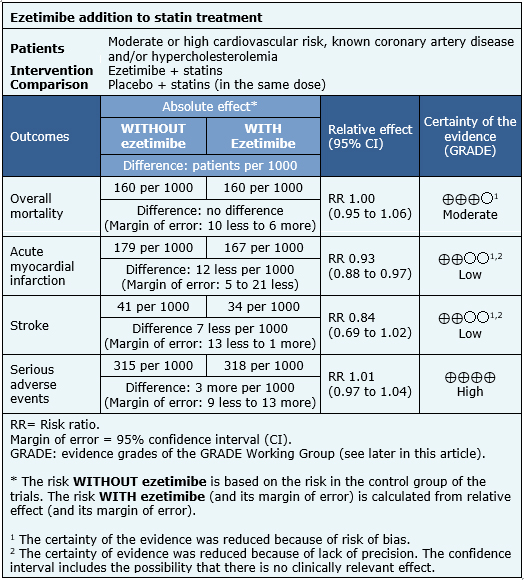
- The addition of ezetimibe to statins probably leads to little or no difference in overall mortality. The certainty of the evidence is moderate.
- The addition of ezetimibe to statins might lead to a small reduction in the risk of myocardial infarction, but the certainty of the evidence is low.
- The addition of ezetimibe to statins might lead to a small reduction in the risk of stroke, but the certainty of the evidence is low.
- The addition of ezetimibe to statins does not increase serious adverse events. The certainty of the evidence is high.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
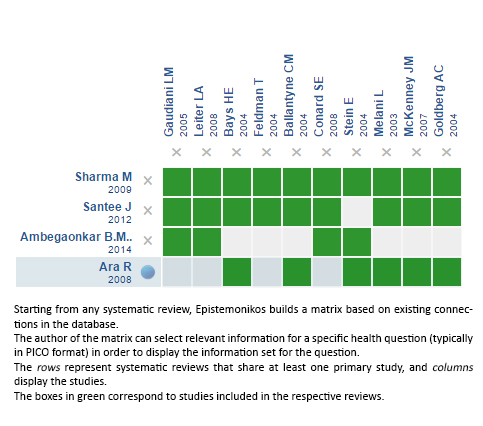
Follow the link to access the interactive version: Ezetimibe for hypercholesterolaemia
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

