Epistemonikos summaries
← vista completaPublished on December 7, 2016 | http://doi.org/10.5867/medwave.2016.6790
Is electroconvulsive therapy during pregnancy safe?
¿Es segura la terapia electroconvulsiva durante el embarazo?
Abstract
Therapeutic options for psychiatric conditions are limited during pregnancy because many drugs are restricted or contraindicated. Electroconvulsive therapy constitutes an alternative, however there is controversy over its safety. Using the Epistemonikos database, which is maintained by searching multiple databases, we found five systematic reviews, including 81 studies overall describing case series or individual cases. Data were extracted from the identified reviews and summary tables of the results were prepared using the GRADE method. We concluded it is not clear what are the risks associated with electroconvulsive therapy during pregnancy because the certainty of the existing evidence is very low. Likewise, existing systematic reviews and international clinical guidelines differ in their conclusions and recommendations.
Problem
Many of the drugs that are used to treat psychiatric conditions have restrictions or even contraindications during pregnancy, which limit the therapeutic options in this group of patients. Electroconvulsive therapy is one of the interventions that are usually considered as a safe and effective alternative for the treatment of severe or resistant conditions during pregnancy, mainly in depression. There is consensus that it is the therapy of choice in such cases, especially when there is a vital risk to the mother or the fetus. However, in less serious cases where this treatment could also be an option to consider, there is controversy, largely because it is unclear what the risks are. On the other hand, the recommendations given by different clinical guidelines differ among them.
Methods
We used Epistemonikos database, which is maintained by screening multiple databases, to identify systematic reviews and their included primary studies. With this information, we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found five systematic reviews [1],[2],[3],[4],[5] which include 81 primary studies overall [6],[7],[8],[9],[10], |
|
What types of patients were included |
Seventeen studies included patients requiring electroconvulsive therapy during the first trimester of pregnancy [12],[13],[16],[19],[30],[34],[36],[38],[44], |
|
What types of interventions were included |
In all studies, patients received electroconvulsive therapy sessions. In two studies [33],[70] reported unilaterally and in 16 studies [11],[22],[23],[24],[25],[35],[38],[50], The total number of sessions ranged from 1 to 35. The timing of electroconvulsive therapy sessions fluctuated between week two of gestation through week 40. The frequency of electroconvulsive therapy was reported in 22 studies [9],[11],[22],[23],[24],[25],[27],[29],[31], |
|
What types of outcomes |
The studies measured multiple outcomes, however, different systematic reviews grouped them as follows:
|
Summary of findings
The information on the use of electroconvulsive therapy during pregnancy is based on 81 studies reporting case series or isolated cases, corresponding to 404 patients. The summary of the results is as follows:
- It is unclear what are the risks associated with electroconvulsive therapy during pregnancy because the certainty of the evidence is very low.
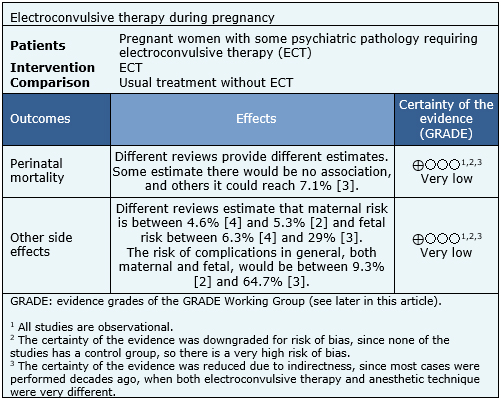

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
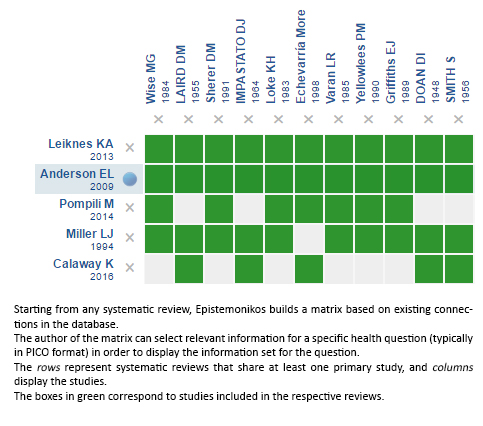
Follow the link to access the interactive version: Electroconvulsive therapy during pregnancy
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

