Epistemonikos summaries
← vista completaPublished on December 14, 2016 | http://doi.org/10.5867/medwave.2016.6795
Do branched chain amino acids improve hepatic encephalopathy in cirrhosis?
¿Aceleran los aminoácidos de cadena ramificada la recuperación de la encefalopatía hepática en pacientes con cirrosis?
Abstract
There is controversy about the effectiveness of branched chain amino acids for treatment of hepatic encephalopathy. Searching in Epistemonikos database, which is maintained by screening multiple databases, we identified seven systematic reviews including 32 randomized controlled trials, of which 30 address the question of this article. We extracted results, combined the evidence using meta-analysis and generated a summary of findings following the GRADE approach. We concluded branched chain amino acids might improve hepatic encephalopathy, but they probably lead to little or no effect on mortality.
Problem
Hepatic encephalopathy is a brain dysfunction associated to the presence of portal-systemic shunting, generally as consequence of liver insufficiency. The pathogenesis of hepatic encephalopathy is not completely understood, but it is accepted hyperammonaemia plays a central role, so most interventions for this condition are directed to a reduction of ammonia.
It is postulated the plasma ratio of aromatic and branched chain amino acids is altered in this condition, leading to an imbalance in neurotransmitter synthesis and accumulation of false neurotransmitters, which would contribute to hepatic encephalopathy.
Therefore, supplementation with branched chain amino acids could improve hepatic encephalopathy. However, it is unclear whether this is an effective intervention.
Methods
We used Epistemonikos database, which is maintained by screening more than 30 databases, to identify systematic reviews and their included primary studies. With this information, we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found seven systematic reviews [1],[2],[3],[4],[5], |
|
What types of patients were included |
In 27 trials, all of the participants had cirrhosis |
|
What types of interventions were included |
Nine trials administered intravenous branched chain amino acids [8],[13],[30],[31],[32],[36],[46],[50],[56], 18 trials used oral branched chain amino acids [16],[20],[21],[24], |
|
What types of outcomes |
The main outcomes addressed by the different systematic reviews were the following:
Other outcomes evaluated were: development of ascites, resolution of ascites, gastrointestinal bleeding, resolution of encephalopathy, infections, bilirubin level, length of hospital stay, stay in intensive care unit, postoperative complications, intra-abdominal complications, postoperative pneumonia, operative wound infection, nitrogen balance, re-hospitalisation post liver transplantation,infections, changes in grade of hepatic encephalopathy, side effects (vomiting, diarrhoea), and time on mechanical ventilation. |
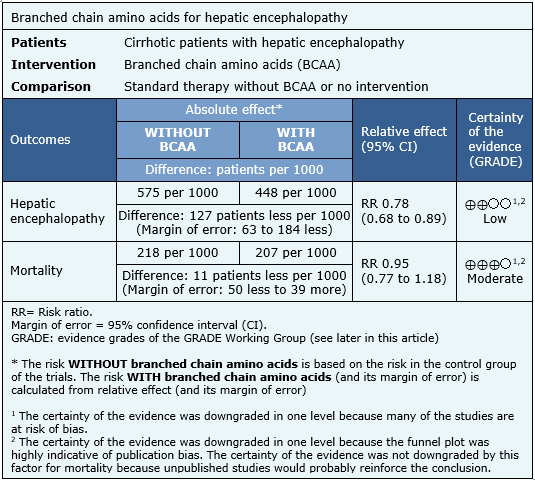
Summary of findings
The information on the effects of branched chain amino acids in patients with hepatic encephalopathy is based on 23 randomized trials [8], [13],[16],[20],[21],[24],[26],[27],[30],[31],[32],[36],[39],[44],[46],[48],[50],[56],[58],[59],[60],[61],[62] including 1040 patients. The remaining trials did not provide data about relevant outcomes, or these were not suitable for meta-analysis. All of the trials measure the outcome hepatic encephalopathy and 19 trials (936 patients) measured mortality [8],[13],[16],[20],[21],[26],[27],[30],[32],[36],[39],[44],[46],[48],[50],[56],[58],[61],[62]. The summary of findings is the following:
- Branched chain amino acids probably lead to little or no effect on mortality in hepatic encephalopathy. The certainty of the evidence is moderate.
- Branched chain amino acids might decrease hepatic encephalopathy, but the certainty of this evidence is low.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
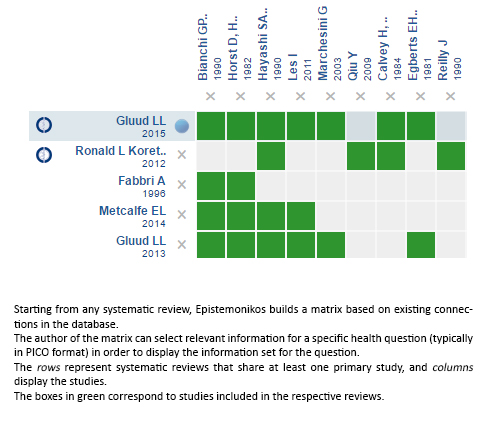
Follow the link to access the interactive version: Branched-chain amino acids for treatment of hepatic encephalopathy
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

