Epistemonikos summaries
← vista completaPublished on December 16, 2016 | http://doi.org/10.5867/medwave.2016.6799
Are leukotriene inhibitors useful for bronchiolitis?
¿Tienen utilidad los inhibidores de leucotrienos en bronquiolitis?
Abstract
Bronchiolitis is a prevalent disease in children under two years of age, which carries significant morbidity and mortality. However, there is controversy regarding the optimal therapeutic management. Leukotriene inhibitors have been proposed as an alternative, although its efficacy is not clear yet. Searching in Epistemonikos database, which is maintained by screening multiple databases, we identified two systematic reviews comprising six trials addressing the question of this article. We extracted data, combined the evidence using meta-analysis and generated a summary of findings following the GRADE approach. We concluded leukotriene inhibitors might not decrease mortality levels on bronchiolitis patients and it is not clear whether they decrease length of hospital stay. They might reduce recurrent wheezing, but the certainty of the evidence is low, and they increase adverse effects.
Problem
Bronchiolitis is characterized by low respiratory tract inflammation due to a viral infection. It mainly affects children under two years of age, especially in winter, and demands health services, leads to high hospitalization rates, ventilation requirements and even mortality. In addition, a higher incidence of recurrent wheezing has been observed in these patients. In general, there is controversy regarding the management of bronchiolitis. Among the therapeutic alternatives, the efficacy of leukotriene inhibitors has been proposed, since the pathogenesis of bronchiolitis involves the stimulation of the enzyme lipoxygenase-5, which participates on leukotriene synthesis. These molecules have been identified as contributors to airway inflammation, airway and alveolar obstruction, mucosal edema and increased bronchial reactivity.
The present summary seeks to evaluate whether leukotriene inhibitor therapy is a useful and safe alternative for the management of pediatric patients with bronchiolitis.
Methods
We used Epistemonikos database, which is maintained by screening multiple databases, to identify systematic reviews and their included primary studies. With this information, we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found two systematic reviews [1],[2], including six randomized controlled trials, reported in 12 references [3],[4],[5],[6],[7],[8],[9],[10],[11],[12],[13],[14]. |
|
What types of patients were included |
The six trials only included patients diagnosed with bronchiolitis [3],[4],[6],[7],[8],[9]. Regarding to age, the six trials included patients younger than 24 months [3],[4],[6],[7],[8],[9]. If this age group is subdivided, one trial included patients between 1 and 24 months [6], two trials included patients between 3 and 24 months [4],[9], one trial included patients between 4 and 24 months [8], one trial included patients between 6 and 24 months [3] and one trial included patients younger than 24 months [7]. Two trials included patients with a first episode of bronchiolitis of any etiology [6],[8], two trials included a first episode of bronchiolitis caused by respiratory syncytial virus [3],[7] and one trial included first or second episode of bronchiolitis caused by respiratory syncytial virus [9]. The six trials only included inpatient population [3],[4],[6],[7],[8],[9]. Of these, two trials required a minimum stay of 24 hours [7], [9]. No trial considered outpatient population. |
|
What types of interventions were included |
The six trials compared oral montelukast versus placebo [3],[4],[6],[7],[8],[9]. Regarding dosing, four trials used 4 mg/day [3],[4],[6],[7], one trial used 8 mg/day [8] and one trial used doses of 4 and 8 mg/day [9]. Regarding intervention duration, two trials administered montelukast since admission day until discharge [6],[8], two trials used it for three months [3],[7], one trial between 1 and 4 weeks [4] and one trial between 4 and 20 weeks [9]. |
|
What types of outcomes |
The systematic reviews reported the following outcomes: All cause-mortality, incidence of recurrent wheezing, length of hospital stay, clinical adverse effects, percentage of children requiring ventilation, percentage of symptom-free days, frequency of recurrent wheezing, serum eosinophil-derived neurotoxin levels, corticosteroids usage, clinical severity score, oxygen saturation and respiratory rate. Follow-up was one year in two trials [3], [7], six months in one [9] and 18 months in one [4]. In two trials the follow-up was not reported [6],[8]. |
Both trials evaluated the use of tetrahydrocannabinol capsules administered orally. In one trial, the dose was 5 mg, 7.5 mg or 10 mg once [9], and in the other trials, the dose was not specified [8].
Summary of findings
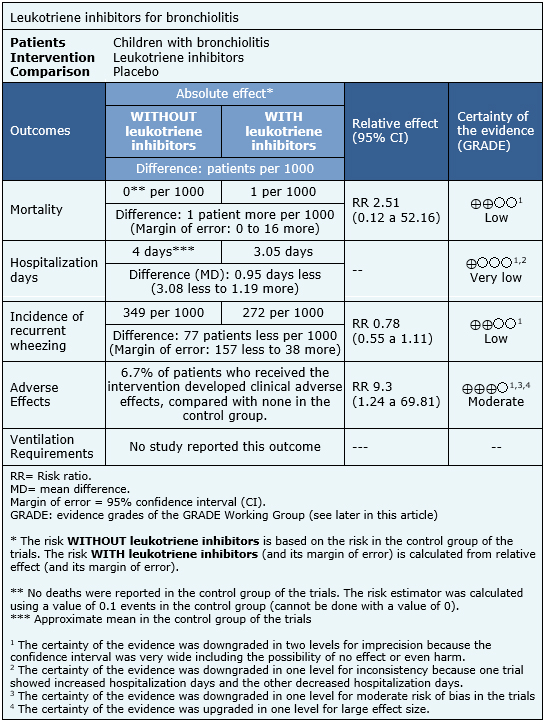
The information regarding the effects of leukotriene inhibitors for bronchiolitis is based on six randomized trials [3],[4],[6],[7],[8],[9]. Only one trial [9] reported mortality (952 patients), two trials [6],[8], reported length of hospital stay (136 patients), two trials [4],[7], reported adverse effects (328 patients) and three trials reported the incidence of recurrent wheezing (1160 patients). No trial assessed ventilation requirements.
The summary of findings is the following:
- Leukotriene inhibitors might not decrease mortality in bronchiolitis, but the certainty of the evidence is low.
- It is not clear whether leukotriene inhibitors decrease hospitalization length in bronchiolitis, because the certainty of the evidence is very low.
- Leukotriene inhibitors might decrease recurrence of wheezing in bronchiolitis, but the certainty of the evidence is low.
- Leukotriene inhibitors increase clinical adverse effects in patients with bronchiolitis, although the magnitude of this increase is uncertain. The certainty of the evidence is moderate.
- None of the studies found evaluated the effect of leukotriene inhibitors on ventilation requirements.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
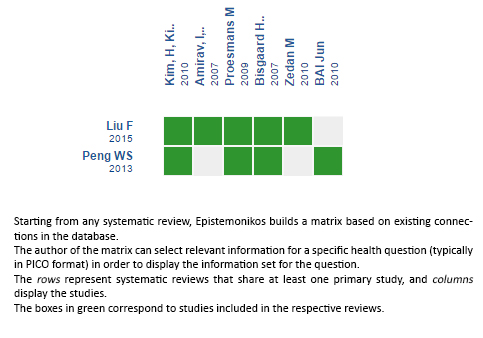
Follow the link to access the interactive version: Leukotriene inhibitors for bronchiolitis
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

