Epistemonikos summaries
← vista completaPublished on December 23, 2016 | http://doi.org/10.5867/medwave.2016.6807
Are probiotics effective to prevent traveler’s diarrhea?
¿Son efectivos los probióticos para prevenir la diarrea del viajero?
Abstract
La diarrea aguda es la enfermedad más común que afecta a los viajeros, principalmente aquellos que se dirigen a regiones de alto riesgo. El uso de probióticos podría prevenir su aparición, sin embargo, los datos que apoyan su uso no son consistentes y no se recomiendan en las guías clínicas actuales. Utilizando la base de datos Epistemonikos, la cual es mantenida mediante búsquedas en múltiples bases de datos, identificamos cuatro revisiones sistemáticas que en conjunto incluyen siete estudios aleatorizados pertinentes a esta pregunta. Realizamos un metanálisis y tablas de resumen de los resultados utilizando el método GRADE. Concluimos que los probióticos podrían prevenir la diarrea del viajero, pero la certeza de la evidencia es baja.
Problem
Acute diarrhea is the most common disease that affects travelers heading to at-risk areas. Although preventive measures related to hygiene have reduced the risk in many destinations, this is still high in some others [1].
The use of probiotics as prophylaxis for traveler's diarrhea seems attractive because of their beneficial effects over intestinal flora, and the reduction in colonization by pathogenic bacteria, in addition to their safety. However, data supporting their use are not consistent [2].
Methods
We used Epistemonikos database, which is maintained by screening multiple databases, to identify systematic reviews and their included primary studies. With this information, we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found four systematic reviews [3],[4],[5],[6], that included seven randomized controlled trials [7],[8],[9],[10],[11],[12],[13]. |
|
What types of patients were included |
Three studies only included adults [7],[11],[13]. One study included patients aged 10 to 80 years [10]. In three studies, the age range was not specified [8],[9],[12]. The included patients traveled to different destinations: |
|
What types of interventions were included |
The trials used different probiotics and in different doses:
All of the trials compared against placebo or standard treatment. |
|
What types of outcomes |
The outcome was the development of diarrhea during the trip, defined as more than three stools per day for at least two days, or more than five stools in 48 hours. |
Both trials evaluated the use of tetrahydrocannabinol capsules administered orally. In one trial, the dose was 5 mg, 7.5 mg or 10 mg once [9], and in the other trials, the dose was not specified [8].
Summary of findings
The information on the effects of probiotics to prevent traveler's diarrhea is based on seven randomized trials including 4,025 patients. All of the trials measured the outcome of diarrhea during the trip, defined as more than three stools per day for at least two days or more than five stools in 48 hours.
The summary of findings is as follows:
- Probiotics might prevent traveler’s diarrhea but the certainty of the evidence is low.
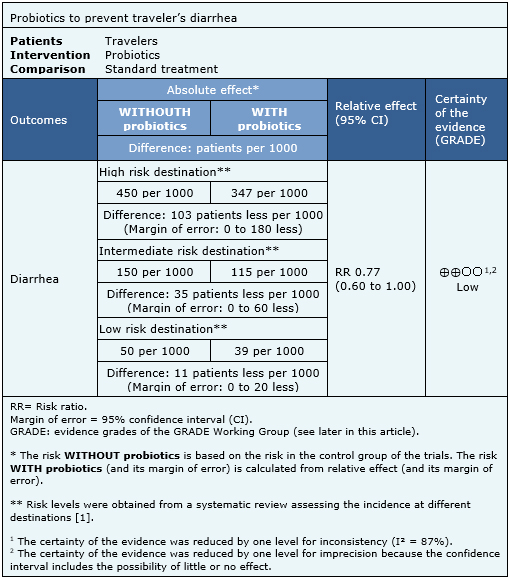
|
Follow the link to access the interactive version of the Summary of Findings (iSoF) table |

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
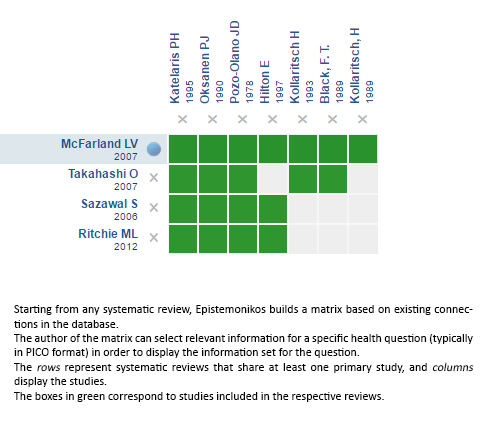
Follow the link to access the interactive version: Probiotics for prevention of traveler's diarrhea
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

