Epistemonikos summaries
← vista completaPublished on January 17, 2017 | http://doi.org/10.5867/medwave.2017.6844
Is pirfenidone effective for idiopathic pulmonary fibrosis?
¿Es efectiva la pirfenidona en el tratamiento de la fibrosis pulmonar idiopática?
Abstract
Idiopathic pulmonary fibrosis has an ominous prognosis and there are virtually no effective therapies. It has been suggested that pirfenidone, an antifibrotic agent, could change its course. Searching in Epistemonikos database, which is maintained by screening multiple databases, we identified 13 systematic reviews comprising nine trials addressing the question of this article, seven of which are randomized and whose results were analyzed in this summary. We combined the evidence using meta-analysis and generated a summary of findings following the GRADE approach. We concluded pirfenidone decreases disease progression and mortality in idiopathic pulmonary fibrosis. Although it is associated with frequent gastrointestinal and cutaneous adverse effects, these are generally not severe.
Problem
Idiopathic pulmonary fibrosis is a disease of low frequency and of uncertain etiology [1]. Its prognosis is similar to lung cancer and until the last decade no intervention had demonstrated survival benefits [2]. After diagnosis, survival decreases rapidly and several factors, including exacerbations, time to disease progression and deterioration in respiratory function are associated with poor prognosis [3],[4].
Pirfenidone [5-methyl-1-phenyl-2- [1H] -pyridone) is an oral antifibrotic, antioxidant and anti-inflammatory drug whose mechanism is based on acting as a scavenger of free hydroxyl radicals (OH-) and superoxide anions (O-), leading to decreased cytokine production (TNF-alpha, IFN-gamma, IL-1Beta, IL-6) and inhibiting fibroblast proliferation [5]. In 1999 the first published trial showed improvement in functional capacity, exacerbations and survival, after which other trials have been conducted [6],[7], which led to the FDA's approval in 2014 for its use in the treatment of idiopathic pulmonary fibrosis [8].
Methods
We used Epistemonikos database, which is maintained by screening multiple databases, to identify systematic reviews and their included primary studies. With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found 13 systematic reviews reported in 14 references [2], [9],[10],[11],[12],[13],[14],[15],[16],[17],[18], [19],[20],[21] comprising nine primary studies, reported in 10 references [6],[22],[23],[24],[25],[26],[27],[28], [29],[30], including seven randomized controlled trials published in eight references [22],[23],[24],[25],[26],[27],[29],[30]. Two randomized trials [30],[31] were not considered in this summary because the study population did not correspond to that of the question under investigation. This table and the overall summary are based on the five randomized trials relevant to the question. |
|
What types of patients were included |
All trials included patients diagnosed with idiopathic pulmonary fibrosis according to the standards of the American Thoracic Society of 2011. Patients ranged from 20 to 80 years of age. One trial included patients with PaO2 ≥ 70 mmHg at rest and SpO2 ≤ 90% on exercise [25], two trials included patients diagnosed with pulmonary fibrosis in the past year [24],[25], while three others included them if the diagnosis had been made in the past four years [22],[23],[26],[27]. Three trials included patients with functional capacity between 50 and 90% [22],[23],[26],[27], three trials included patients with DLCO> 30% [22],[23],[26],[27], two trials included patients with walk test in six minutes higher or equal to 150 m [23],[26],[27] and one trial included patients with FEV1 / FVC> 70%. [22]. |
|
What types of interventions were included |
All trials used pirfenidone as monotherapy. Three trials used pirfenidone at doses of 2,400 mg daily [22],[23],[26],[27] and two trials used pirfenidone at doses of 1,800 mg daily [24],[25].Two trials administered concomitant treatment with prednisolone at doses of less than 10 mg daily [24],[25]. All trials compared against placebo. |
|
What types of outcomes |
The different systematic reviews identified grouped the outcomes as follows:
|
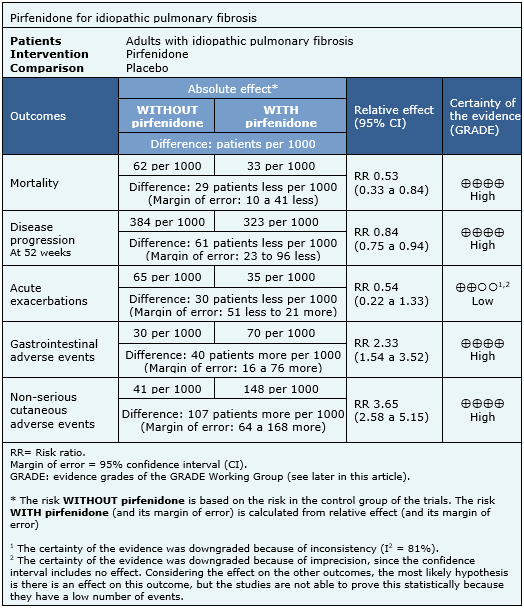
Summary of findings
The information on the effects of pirfenidone is based on five randomized trials [22],[23],[24],[25],[26],[27] involving 1,567 patients. All trials reported mortality, two trials [24],[25] reported acute exacerbations and four trials [22],[23],[24],[26],[27] reported progression-free survival. The summary of findings is as follows:
- Pirfenidone decreases mortality in idiopathic pulmonary fibrosis. The certainty of the evidence is high.
- Pirfenidone decreases disease progression in idiopathic pulmonary fibrosis. The certainty of the evidence is high.
- Pirfenidone might reduce the risk of acute exacerbations, but the certainty of the evidence is low.
- Pirfenidone has frequent, but not severe, gastrointestinal side effects. The certainty of the evidence is high.
- Pirfenidone has frequent, but not severe, cutaneous adverse effects. The certainty of the evidence is high.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
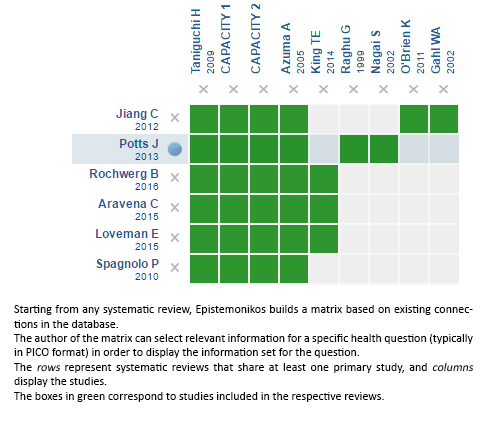
Follow the link to access the interactive version: Pirfenidone for idiopathic pulmonary fibrosis
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

