Epistemonikos summaries
← vista completaPublished on March 21, 2017 | http://doi.org/10.5867/medwave.2017.6871
Is primary prevention with antiepileptic drugs effective in brain tumors or brain metastases?
¿Es efectiva la prevención primaria con anticonvulsivantes en tumores o metástasis cerebrales?
Abstract
Patients with brain tumors –primary or metastatic- have an increased risk of presenting seizures during the course of their disease. So, prophylactic antiepileptic drugs have been proposed. However, the effects of this intervention are not yet clear. To answer this question, we searched in Epistemonikos database, which is maintained by screening multiple databases. We identified 12 systematic reviews including 80 studies overall. Twelve corresponded to randomized trials, but only two answered the question of interest. We extracted data, conducted a meta-analysis and generated a summary of findings table using the GRADE method. We concluded primary prevention with antiepileptic drugs might not reduce the risk of seizures, and it is associated to frequent adverse effects.
Problem
Up to 60% of patients with brain tumors develop seizures. Various mechanisms have been proposed for this; neoplastic tissue could be the starting site of a seizure, especially if it is neural tissue, and on the other hand, intracranial lesions could alter both structurally and functionally the adjacent territory by causing edema, vascular insufficiency, inflammation or releasing metabolically active molecules that promote epileptic activity. It is proposed that the location of the lesions could also influence the onset of seizures, being the incidence higher in cortical lesions.
This summary seeks to answer if primary prevention with antiepileptic drugs is effective in patients that will not be subject to surgery.
Methods
We used Epistemonikos database, which is maintained by screening multiple databases, to identify systematic reviews and their included primary studies. With this information, we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found 12 systematic reviews [1],[2],[3],[4],[5],[6], [7],[8],[9],[10],[11],[12] that include 80 primary studies [13],[14],[15],[16],[17],[18],[19],[20],[21],[22],[23], [24],[25],[26],[27],[28],[29],[30],[31],[32],[33],[34], [35],[36],[37],[38],[39],[40],[41],[42],[43],[44],[45], [46],[47],[48],[49],[50],[51],[52],[53],[54],[55],[56], [57],[58],[59],[60],[61],[62],[63],[64],[65],[66],[67], [68],[69],[70],[71],[72],[73],[74],[75],[76],[77],[78], [79],[80],[81],[82],[83],[84],[85],[86],[87],[88],[89], [90],[91],[92], twelve of which are randomized controlled trials [13],[14],[17],[22],[23],[36],[42],[65],[84],[86],[91],[92]. Ten of the latter were not used for analyses in this summary because they evaluated patients that would be subject to surgery [17],[22],[23],[36],[42],[65],[84], [86],[91],[92], so only two randomized trials correspond to direct evidence and answer our question [13],[14]. |
|
What types of patients were included |
Both trials included patients older than 18 years of age, without any previous history of seizures, with diagnosis of primary brain tumor (28.2%) or cerebral metastases (71.8%) and who were not subject to surgery. None of the trials reported the location of the lesions. One trial [13] included people with less than a month from diagnosis, and the other trial [14] included patients with less than 14 days from the time of diagnosis. No trial reported the treatment for the underlying disease. |
|
What types of interventions were included |
One trial [13] administered for primary prevention 15 mg/kg of phenytoin orally as an initial dose, followed by 5mg/kg/day orally. They followed the patients for 5.4 months. One trial [14] used valproate with a target serum level of 50-100 μg/mL, and followed the patients for seven months. One trial compared the intervention against placebo [14] and the other did not give any treatment to the control group [13]. |
|
What types of outcomes |
The main outcome reported was the incidence of seizures. Other reported outcome was the incidence of adverse effects such as nausea, vertigo, myelosuppression, blurred vision, rash and ataxia. No trial evaluated quality of life or mortality. |
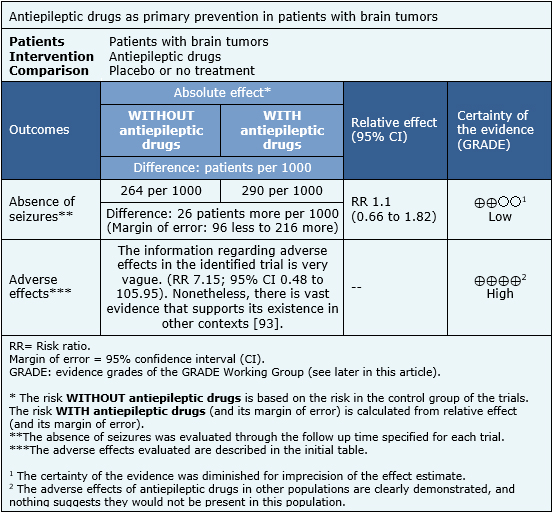
Summary of findings
The information regarding the effects of antiepileptic drugs for primary prevention in brain tumors or brain metastases is based on two randomized trials [13],[14], which include 174 patients. Both trials measured the onset of seizures and the presence of adverse effects. No trial evaluated mortality or quality of life.
The summary of findings is:
- Primary prevention with antiepileptic drugs might not decrease the risk of seizures in patients with brain tumors or cerebral metastases, but the certainty of the evidence is low.
- The use of antiepileptic drugs is associated to frequent adverse affects. The certainty of the evidence is high.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
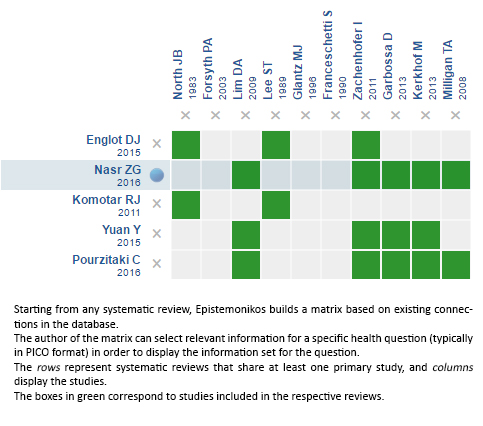
Follow the link to access the interactive version: Antiepileptic drugs for patients with brain tumors
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

