Epistemonikos summaries
← vista completaPublished on March 24, 2017 | http://doi.org/10.5867/medwave.2017.6873
Is a short-course antibiotic treatment effective for streptococcal tonsillopharyngitis in children?
¿Es efectivo un esquema antibiótico acortado para el tratamiento de la faringoamigdalitis bacteriana en niños?
Abstract
Acute bacterial tonsillopharyngitis in children has been classically treated with long courses of antibiotic, usually 10 days, with the intention to prevent the occurrence of complications. However, it has not been clarified whether a shortened treatment could be equally effective in fulfilling that purpose. To answer this question, we searched in Epistemonikos database, which is maintained by screening multiple databases. We identified five systematic reviews including 59 randomized trials overall. We extracted data, conducted a meta-analysis and generated a summary of findings table using the GRADE approach. We concluded that a shortened antibiotic regimen is probably similar, or with minimal differences, to a longer course, and might not make any difference regarding complications related to Streptococcus group A infection.
Problem
Traditionally, bacterial tonsillopharyngitis in children, mainly caused by Streptococcus, has been treated with long antibiotic courses (10 days) with the purpose of eradicating Streptococcus group A and consequently preventing its complications. However, it is unknown whether a shortened treatment can be equally effective to accomplish that same purpose, probably reducing costs, possible adverse effects and improving adherence to treatment.
Methods
We used Epistemonikos database, which is maintained by screening multiple databases, to identify systematic reviews and their included primary studies. With this information, we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found five systematic reviews [1],[2],[3],[4],[5] including 59 primary studies, reported in 54 references (some articles report more than one study) [6],[7],[8], [9],[10],[11],[12],[13],[14],[15],[16],[17],[18],[19],[20], [21],[22],[23],[24],[25],[26],[27],[28],[29],[30],[31], [32],[33],[34],[35],[36],[37],[38],[39],[40],[41],[42], [43],[44],[45],[46],[47],[48],[49],[50],[51],[52],[53], [54],[55],[56],[57],[58],[59]. All of the studies correspond to randomized controlled trials. We excluded from the analysis those trials whose long course of antibiotics lasted less than 10 days [33],[58], [59]. We included trials that involved only pediatric patients, whose age was 18 years or less [6],[7],[8],[9],[10],[12], [13],[14],[15],[19],[21],[23],[24],[25],[30],[31],[32], [37],[41],[43],[44],[45],[46],[48],[49],[50],[52],[53], [56],[57], and trials that enrolled adults too, if they were analyzed independently [34]. That is, 36 randomized trials, reported in 31 references. |
|
What types of patients were included |
Patients who participated in the trials included in this review correspond in their entirety to pediatric patients (between six months and 18 years). All trials included only patients with clinically compatible symptoms and microbiological diagnosis of Streptococcus group A (by culture or rapid test). One trial also included Anti-streptolysin O antibodies (ASO) as a possible test for the microbiological certification of Streptococcus group A [34]. |
|
What types of interventions were included |
All included trials compared the use of shortened antibiotic treatment (three to seven days) versus a standard 10-day antibiotic treatment. Only five trials [15],[24],[34],[37],[46] used the same antibiotic as standard and shortened treatment. One of these used cefaclor [15], one cefuroxime [24], one cefetamet [34], one cefpodoxime [37] and one penicillin V [46]. Nineteen trials used a macrolide as a shortened treatment; sixteen compared it to a beta-lactam [13],[14],[19],[23], [30],[31],[41],[43],[44],[48],[49],[50],[52] and three to a macrolide [32],[56],[57]. Sixteen trials used a beta-lactam as a shortened treatment; all were compared to a beta-lactam [6],[7], [8],[10],[12],[15],[21],[24],[25],[34],[37],[45],[46],[52], [53]. One trial compared the use of a macrolide or beta-lactam versus a standard penicillin treatment [9]. One trial allowed the use of analgesics as cointervention [15], two did not allow the use of concomitant analgesics [23],[34], and the rest did not report cointerventions. In all trials, except one [34], an early follow-up was conducted between days 0 to 15 once completed the antibiotic treatment. The majority [6],[7],[9],[10],[12], [13],[19],[21],[23],[25],[30],[31],[37],[41],[43],[44], [45],[52],[53],[56] also report having a late follow-up of variable duration with up to one year of follow-up after treatment. |
|
What types of outcomes |
The identified systematic reviews grouped the outcomes as follows:
|
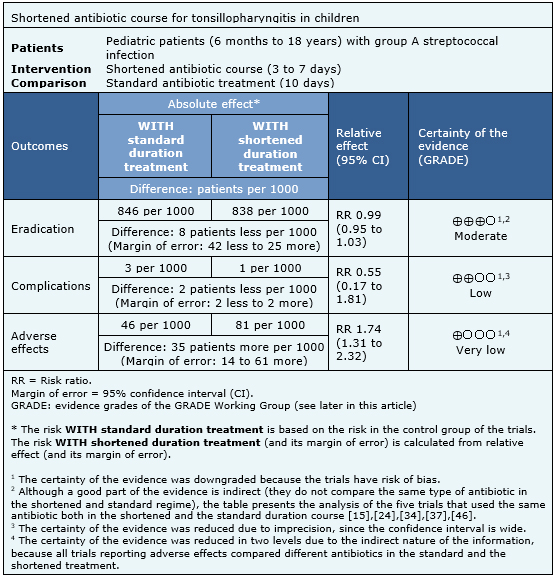
Summary of findings
Information on the effects of shortened treatment of bacterial tonsillopharyngitis in children is based on 36 randomized trials. All of the trials (15,869 patients) reported microbiological eradication; three trials (8,135 patients) complications of group A Streptococcus [9],[44],[45] and 21 trials [6],[7],[10],[12],[13],[19],[21],[23],[29],[30],[31],[37],[41],[43],[44],[45],[52],[53] (7,997 patients) measured the adverse effects of therapy. The summary of findings is as follows:
- A shortened antibiotic course probably results in little or no difference in the Streptococcus group A eradication rate for bacterial tonsillopharyngitis in children, compared to a longer course. The certainty of the evidence is moderate.
- A shortened antibiotic course might not make any difference in the complications related to Streptococcus group A infection, compared to a longer course. The certainty of the evidence is low.
- It is unclear whether a shortened antibiotic course reduces adverse effects because the certainty of the evidence is very low.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
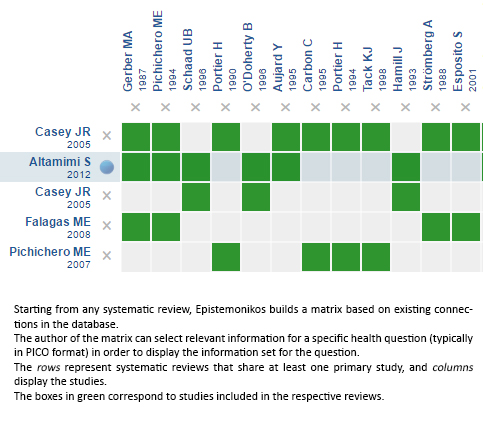
Follow the link to access the interactive version: Short-course antibiotic treatment for tonsillopharyngitis
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

