Epistemonikos summaries
← vista completaPublished on May 12, 2017 | http://doi.org/10.5867/medwave.2017.6942
Sevelamer versus calcium-based phosphate binders for chronic kidney disease
Sevelamer comparado con quelantes de fósforo en base a calcio para la insuficiencia renal crónica
Abstract
Chronic kidney disease-mineral and bone disorder is prevalent. There is controversy regarding whether calcium-based phosphate binders or sevelamer - a non-calcium phosphate binder – constitute a better therapeutic alternative. Searching in Epistemonikos database, which is maintained by screening multiple information sources, we identified 12 systematic reviews comprising 61 studies of which 41 correspond to randomized trials addressing the question of this article. We combined the evidence using meta-analysis and generated a summary of findings following the GRADE approach. We concluded sevelamer may decrease hypercalcemia, but with a higher incidence of gastrointestinal effects than calcium based phosphate binders. It is unclear if there are differences in mortality because the certainty of the evidence is very low.
Problem
Chronic kidney disease – mineral and bone disorder is manifested by one or a combination of the following [1]:
- Abnormalities of calcium, phosphorus, parathyroid hormone or vitamin D metabolism.
- Abnormalities in bone turnover, mineralization, volume, linear growth or strength.
- Vascular or other soft tissue calcification.
Hyperphosphatemia is linked to multiple aspects of CKD-MBD. Guidelines recommend to lower serum phosphorus in patients with hyperphosphatemia with phosphate binders.
There are two main therapeutic alternatives; calcium-based phosphate binders (calcium acetate and calcium carbonate) and non-calcium phosphate binders, in particular sevelamer, a polymer of allylamine hydrochloride. However, there is still controversy regarding which alternative constitute a better option.
Methods
We used Epistemonikos database, which is maintained by screening multiple information sources, to identify systematic reviews and their included primary studies. With this information, we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found 12 systematic reviews reported in 13 references [2],[3],[4],[5],[6],[7],[8],[9],[10],[11],[12],[13],[14] that included 61 primary studies reported in 75 references [15], [16],[17],[18],[19],[20],[21],[22],[23],[24],[25],[26], [27],[28],[29],[30],[31],[32],[33],[34],[35],[36],[37], [38],[39],[40],[41],[42],[43],[44],[45],[46],[47],[48], [49],[50],[51],[52],[53],[54],[55],[56],[57],[58],[59], [60],[61],[62],[63],[64],[65],[66],[67],[68],[69],[71], [71],[72],[73],[74],[75],[76],[77],[78],[79],[80],[81], [82],[83],[84],[85],[86],[87],[88],[89]. Of these, 41 studies (54 references) were randomized trials.This table and the summary in general are based on the latter because observational studies did not increase the certainty of the evidence, or provide additional relevant information. |
|
What types of patients were included* |
Regarding the stage of nephropathy, 27 trials [15],[18], [18],[20],[22],[26],[33],[34],[35],[36],[37],[41],[43], [44],[47],[50],[52],[53],[54],[56],[57],[64],[68],[69], [74],[75],[81],[84] included patients in hemodialysis, three [31],[39],[61] in peritoneal dialysis, nine [23],[27],[29],[38],[46],[63],[73],[85],[88] included patients on stage III or IV without dialysis, one trial [77] did not specify the type of dialysis, only that patients had more than six months in this therapy and one trial [89] only required that they were in stage V**. |
|
What types of interventions were included* |
All of the trials used sevelamer as intervention in dose adjusted to phosphorus levels. It was not possible to extract more detailed dosing data from systematic reviews. Six trials [33],[34],[36],[47],[53],[75] used vitamin D as cointervention by any route of administration, one [20] used oral vitamin D, three [18],[39],[69] used intravenous vitamin D, one trial [68] used atorvastatin and one [77] calcium carbonate in low-dose. Regarding comparison, 14 trials [18],[20],[27],[46],[47], [56],[57],[61],[63],[64],[68],[69],[88],[89] used calcium acetate, 20 trials [15],[26],[29],[31],[36],[37],[38],[39], [41],[43],[50],[52],[53],[54],[73],[74],[75],[77],[84],[85] used calcium carbonate, and five [22],[33],[35],[44],[81] used calcium carbonate and calcium acetate. |
|
What types of outcomes |
The outcomes were pooled by the different systematic reviews as follows:
|
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
** Stage of chronic renal failure according to MDRD-4.
Summary of findings
The information on the effects of sevelamer compared to calcium-based phosphate binders is based on all trials included in this summary, which include 5928 patients.
Thirty-one randomized trials [18],[20],[22],[23],[26],[27],[29],[33],[36],[37],[38],[39],[41],[47],[50],[53],[54],[56],[57],[61],[63],[68],[69],[73],[74],[75],[81],[84],[85],[88],[89] reported mortality including 5011 patients. Twenty trials [20],[23],[27],[29],[33],[35],[39],[41],[43],[50],[53],[54],[56],[57],[63],[68],[74],[75],[81],[88] reported gastrointestinal adverse events including 2863 patients. Nineteen trials [20],[22],[23],[26],[27],[33],[36],[38],[39],[43],[47],[56],[57],[68],[69],[74],[75],[81],[88] reported hypercalcemia including 2867 patients.
The summary of findings is the following:
- Sevelamer compared to calcium-based phosphate binders might decrease hypercalcemia events. The certainty of the evidence is low.
- Sevelamer compared to calcium-based phosphate binders might increase the incidence of gastrointestinal effects. The certainty of the evidence is low.
- It is unclear if there are differences in mortality between sevelamer and calcium-based phosphate binders because the certainty of the evidence is very low.
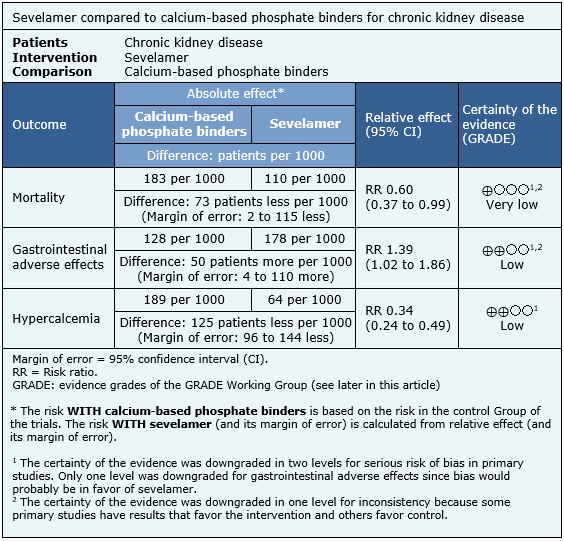
| Follow the link to access the interactive version of the Summary of Findings (iSoF) table |

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
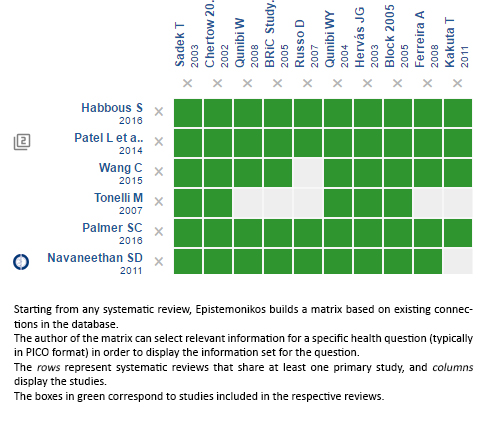
Follow the link to access the interactive version: Sevelamer versus calcium salts for chronic kidney disease
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrices and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

