Epistemonikos summaries
← vista completaPublished on June 16, 2017 | http://doi.org/10.5867/medwave.2017.6974
Are cannabinoids effective for Parkinson’s disease?
¿Son efectivos los cannabinoides en la enfermedad de Parkinson?
Abstract
It is postulated cannabinoids may have benefits in Parkinson's disease. However, its actual clinical effectiveness is still discussed. To answer this question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We identified six systematic reviews including eight studies overall, of which four were randomized trials relevant for the question of interest. We extracted data from the systematic reviews, reanalyzed data of primary studies included in these reviews, conducted a meta-analysis and generated a summary of findings table using the GRADE approach. We concluded cannabinoids probably do not decrease symptoms in Parkinson’s disease or dyskinesia, and probably are associated to frequent adverse effects in patients with Parkinson’s disease.
Problem
The concept of cannabinoids includes organic compounds that interact with cannabinoid receptors in the body. These are in general active metabolites of the cannabis plant, and would explain its pharmacological effects in humans.
Theories to justify the effect of these drugs in Parkinson's disease point to alterations in the cerebral endocannabinoid system, at substantia nigra, caudate and putamen. However, it remains unclear how effective or well tolerated cannabinoids are in Parkinson’s disease.
Methods
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found six systematic reviews [1],[2],[3],[4],[5],[6] including eight studies that answer the question of interest [7],[8],[9],[10],[11],[12],[13],[14] from which four [7],[8],[12],[13] were randomized trials. This table and the summary in general are based on the latter. |
|
What types of patients were included* |
Patients with Parkinson’s disease with or without motor complications, especially dyskinesia, were included. It was not possible to obtain information about the proportion of patients with cognitive impairment or other non-motor manifestations. |
|
What types of interventions were included* |
One trial used extract of Cannabis sativa (tetrahydrocannabinol 2.5 mg with 1.25 mg cannabidiol) [7], one trial used cannabidiol 75 to 300 mg daily [8], one trial used cannabinoid receptor agonist-1 (SR141716) 20 mg daily [12] and other trial used oral nabilone 0.03 mg/kg daily [13]. |
|
What types of outcomes |
From multiple outcome measured by the trials, the systematic reviews grouped them as follows:
Mean follow-up was four weeks, with a range of two to six weeks. |
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
Summary of findings
The information about the effects of cannabinoids for Parkinson’s disease is based on four randomized trials [7],[8],[12],[13] including 63 patients.
None of the systematic reviews presented meta-analysis, so the information presented below corresponds to a narrative synthesis of the information obtained.
The summary of findings is the following:
- Cannabinoids probably do not decrease symptoms of Parkinson’s disease. The certainty of the evidence is moderate.
- Cannabinoids probably do not decrease dyskinesia in Parkinson’s disease. The certainty of the evidence is moderate.
- Cannabinoids are probably associated to adverse effects in patients with Parkinson’s disease. The certainty of the evidence is moderate.
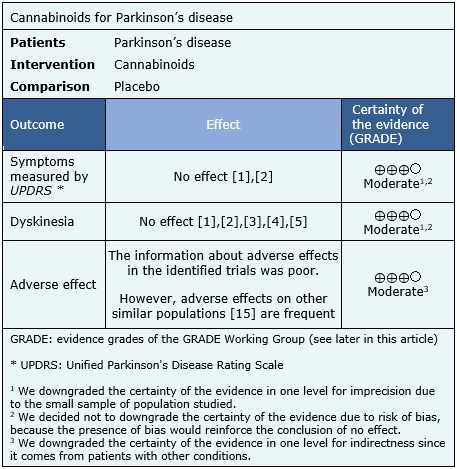
|
Follow the link to access the interactive version of the Summary of Findings (iSoF) table |

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
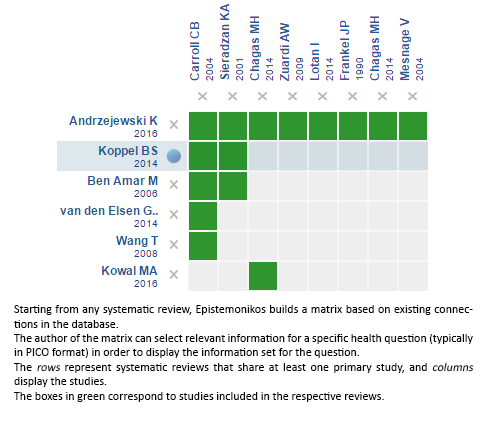
Follow the link to access the interactive version: Cannabinoids for Parkinson’s disease
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrices and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

