Epistemonikos summaries
← vista completaPublished on June 27, 2017 | http://doi.org/10.5867/medwave.2017.6981
Topical corticosteroids or vitamin D analogues for plaque psoriasis?
¿Corticoides tópicos o análogos de vitamina D para la psoriasis en placa?
Abstract
Psoriasis is a frequent chronic inflammatory disease. The plaque variant being its most common form of presentation. Although there is still no cure, treatment alternatives that induce remission and reduce lesions are available. Topical therapies, particularly corticosteroids and vitamin D analogues, are considered effective, but it is still not clear which would be the best alternative. To answer this question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We identified eight systematic reviews including 26 studies overall, of which 22 were randomized trials relevant for the question of interest. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach. We concluded there might be little or no difference in clinical response between topical corticosteroids and topical vitamin D analogues, but topical corticosteroids are less irritating at the site of application. No studies evaluating their long term adverse effects were found.
Problem
Psoriasis is a systemic inflammatory disease, where skin is the most commonly affected organ. Although there is still no cure, there is a wide variety of treatments available that can induce remission and reduce lesions. In daily practice different alternatives are used, and therapy is adjusted according to clinical response. The most commonly used drugs, due to their greater availability, ease of application and lower costs are topical medications. Within these, corticosteroids are the most used. Even though there are many available alternatives such as tazarotene, tacrolimus, anthralin, UV light, among others, vitamin D analogues are frequently chosen. Despite vast experience with these topical therapies, there is stilll controversy about their effects.
Methods
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found eight systematic reviews, reported in nine references [1],[2],[3],[4],[5],[6],[7],[8],[9], that include 26 primary studies, reported in 32 references [10],[11], |
|
What types of patients were included* |
All of the trials included adult patients (15 to 90 years) with plaque psoriasis in trunk and limbs, not including scalp. Trials includes patients with low, moderate and severe disease. |
|
What types of interventions were included* |
All trials used topical corticosteroids as intervention. Within them, some of high potency were used such as fluocinonide 0.05% twice a day [11], betamethasone dipropionate 0.05% once, or twice a day [13],[16],[32], [40], betamethasone 17-valerate 0.1% once [37],[39] or twice a day [15],[23],[26],[29],[41] and desoxymethasone 0.25% twice a day [21]. Also, some corticosteroids of very high potency were used such as clobetasol propionate 0.05% twice a day [22],[24] and diflorasone diacetate 0.05% twice a day [28]. As a comparison, topical treatment with a vitamin D analogue was used, including calcipotriol 50 mcg/g once [17],[20] or twice a day [11],[15],[16],[21],[22],[23],[24],[28],[29],[32],[40],[41], calcitriol 3 mcg/g twice a day [13],[26] and tacalcitol |
|
What types of outcomes |
The outcomes were pooled by the different systematic reviews as follows:
|
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
Summary of findings
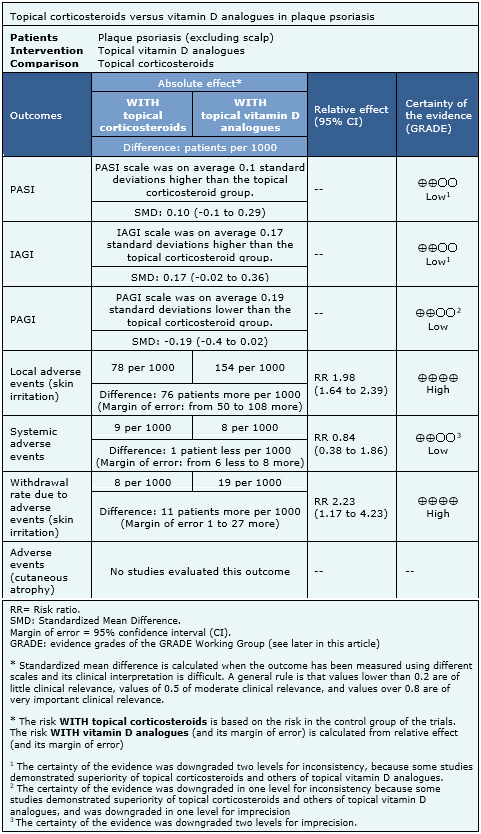
Information on the effects of topical corticosteroids versus vitamin D analogues is based on 15 randomized trials [11],[13],[15],[17],[20],[22],[23],[24],[26],[28],[29],[32],[37],[39],[41] that included 4238 patients overall. Nine trials reported PASI [13],[15],[17],[20],[23],[24],[29],[32],[41], nine reported IAGI [11],[13],[17],[20],[22],
- There might be little or no difference in PASI score between topical corticosteroids and topical vitamin D analogues. The certainty of the evidence is low.
- There might be little or no difference IAGI score between topical corticosteroids and topical vitamin D analogues. The certainty of the evidence is low.
- There might be little or no difference in PAGI score between topical corticosteroids and topical vitamin D analogues. The certainty of the evidence is low.
- Topical corticosteroids lead to fewer local adverse events (skin irritation) than topical vitamin D analogues. The certainty of the evidence is high.
- There might be little or no difference in systemic adverse events between topical corticosteroids and topical vitamin D analogues. The certainty of the evidence is low.
- Topical corticosteroids lead to fewer withdrawals due to adverse events than topical vitamin D analogues. The certainty of the evidence is high.
- No studies were found that evaluated the impact of topical corticosteroids and topical vitamin D analogues in cutaneous atrophy.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
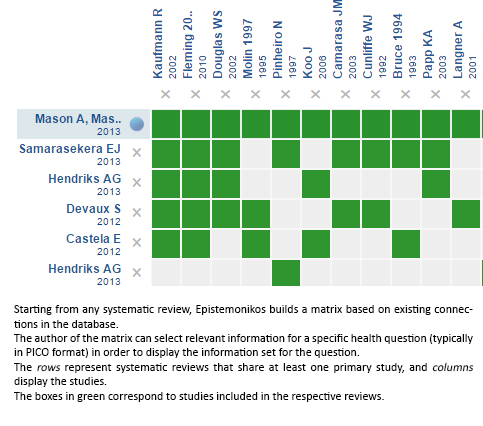
Follow the link to access the interactive version: Topical corticosteroids versus vitamin D analogues in plaque psoriasis excluding scalp psoriasis
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrices and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

