Epistemonikos summaries
← vista completaPublished on August 31, 2017 | http://doi.org/10.5867/medwave.2017.07.7018
Is device-modified trabeculectomy better than classic surgery for treatment of glaucoma?
¿Es mejor la trabeculectomía modificada con dispositivo o la cirugía clásica para el manejo del glaucoma?
Abstract
Several techniques have emerged as complement or replacement for trabeculectomy, the standard surgery for glaucoma. Device-modified trabeculectomy is a recently developed technique whose results compared to the classical technique have not been fully defined. To answer this question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We identified eight systematic reviews including 34 studies overall. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach. We concluded device-modified trabeculectomy probably leads to greater overall success rate and may decrease intraocular pressure more than classical surgery. In addition, this technique would probably have a better safety profile than standard trabeculectomy.
Problem
Glaucoma is the second leading cause of blindness worldwide according to the World Health Organization. Among the known risk factors for the development of this disease is the increase in intraocular pressure (IOP). Trabeculectomy is the standard surgery for patients with uncontrolled glaucoma despite medical treatment. The use of devices has been recently added to the classical surgical technique, in order t promote the flow of aqueous humor from the anterior chamber and to avoid the post-trabeculectomy scaring by maintaining a continuous drainage of aqueous humor. In this line, the use of devices has been proposed as a technique that could improve surgical success and decrease the associated complication rate.
Methods
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found eight systematic reviews [1],[2],[3],[4],[5],[6],[7],[8] that include 48 primary studies reported in 54 references [9],[10],[11],[12],[13],[14],[15],[16],[17],[18], |
|
What types of patients were included* |
The characteristics of included patients were: Regarding the type of glaucoma, 14 trials included patients with open angle glaucoma [12],[18],[19],[21],[25],[34], Regarding the severity of glaucoma, 21 trials included patients with glaucoma refractory to medical treatment. [13],[14],[15],[16],[18],[19],[21],[32],[36],[37],[38],[39], |
|
What types of interventions were included* |
Regarding the type of intervention, 18 trials used amniotic membrane [12],[13],[14],[25],[31],[32],[34],[36],[37], Regarding the use of mitomycin C (MMC), 15 trials did not use MMC in any of their arms [13],[14],[19],[21],[25], All trials compared versus standard treatment (trabeculectomy). |
|
What types of outcomes |
The systematic reviews grouped the outcomes as follows:
|
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
[48],[53],[54],[57],[58],[59],[60],[61],[62]Summary of findings
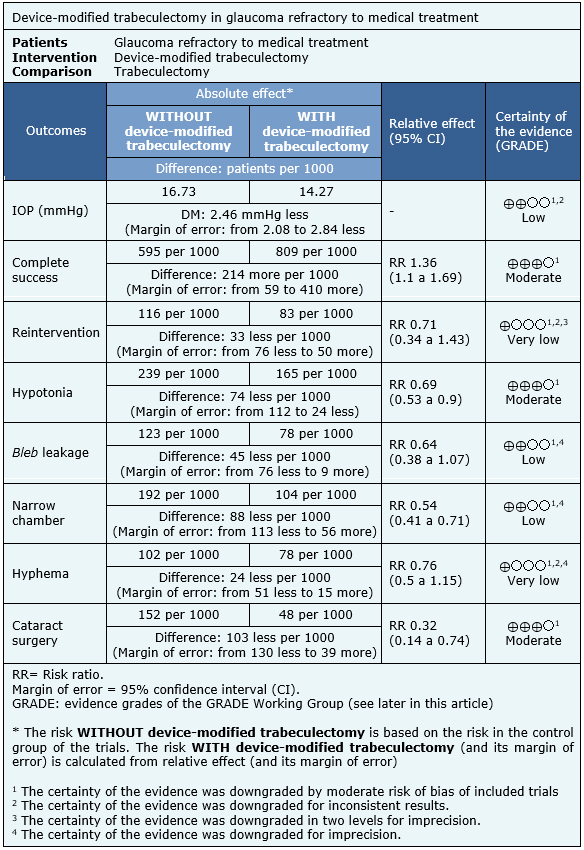
The information on the effects of device-modified trabeculectomy is based on 34 randomized trials including 1540 eyes.
Twenty-five trials measured absolute decrease in intraocular pressure (1164 eyes) [13],[14],[15],[16],[18],[21],[25],[31],[32],[34],[36],[37],[39],[42],[45],[48],[52],[53],[56],[57],[58],[59],[60],[61],[62], three trials assessed complete success (151 eyes) [18],[21],[56], six trials measured the need for reintervention (258 eyes) [18],[21],[39],[45],[49],[56], 14 trials measured hypotonia (695 eyes) [15],[18],[21],[34],[37],[42],[43],[45],[48],[49],[50],[52],[56],[62], 10 trials measured bleb filtration (521 eyes) [14],[16],[18],[21],[43],[48],[50],[52],[56],[62], 23 trials measured narrow chamber (1199 eyes) [14],[15],[18],[21],[25],[31],[32],[34],[36],[39],[42],[43],[48],[49],[50],[52],[56],[57],[58],[59],[60],[61],[62], 16 trials measured hyphema (818 eyes) [13],[14],[15],[16],[18],[21],[39],[43],[45],[49],[50],[52],[53],[56],[58],[60] and 3 trials measured the need for cataract surgery (264 eyes) [21],[43],[56].
The summary of findings is as follows:
- Device-modified trabeculectomy may decrease intraocular pressure more than standard surgery. The certainty of the evidence is low
- Device-modified trabeculectomy probably achieves greater complete success than standard surgery. The certainty of the evidence is moderate.
- It is not clear whether device-modified trabeculectomy decreases reintervention, because the certainty of the evidence is very low.
- Device-modified trabeculectomy probably decreases hypotonia. The certainty of the evidence is moderate.
- Device-modified trabeculectomy may decrease bleb leakage. The certainty of the evidence is low.
- Device-modified trabeculectomy may decrease narrow chamber. The certainty of the evidence is low.
- It is not clear whether device-modified trabeculectomy reduces hyphema, because the certainty of the evidence is very low.
- Device-modified trabeculectomy is likely to decrease cataract surgery. The certainty of the evidence is moderate.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
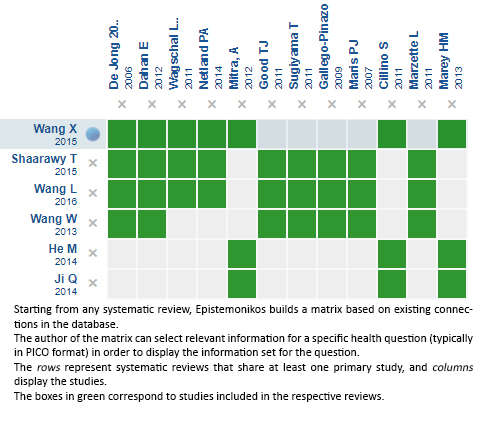
Follow the link to access the interactive version: Device modified trabeculectomy in glaucoma
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrices and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

