Epistemonikos summaries
← vista completaPublished on October 12, 2017 | http://doi.org/10.5867/medwave.2017.08.7041
Is diacerein an alternative for the treatment of osteoarthritis?
¿Es la diacereína una alternativa para el tratamiento de la artrosis?
Abstract
INTRODUCTION: Among the possibilities for the management of osteoarthritis, different pharmacological alternatives have been proposed, being diacerein one of them due to its anti-inflammatory effect. However, diacerein clinical utility is not clear. METHODS: To answer this question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach. RESULTS AND CONCLUSION: We concluded diacerein probably leads to a slight reduction in pain, but would not improve functionality among patients with knee osteoarthritis, and can frequently present diarrhea as an adverse effect.
Problem
Osteoarthritis is a chronic joint disease of high prevalence, especially in older adults. Pain is one of the main symptoms and the major determinant of loss of function. Many patients remain symptomatic despite standard treatment. One of the therapeutic options that has been proposed is the use of diacerein, an anti-inflammatory with inhibitory activity on interleukin-1 beta. Diacerein would reduce IL-1 receptors levels, which would be involved in the destruction of articular cartilage through the inhibition of matrix protein synthesis and increased secretion of proteases in vitro. However, this drug is also associated with adverse effects, mainly diarrhea, so it is not clear if it is effective and if benefits are greater than risks.
Methods
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found five systematic reviews [1],[2],[3],[4],[5] which include nine primary studies [6],[7],[8],[9],[10],[11],[12],[13],[14] which answer the question of interest. All studies correspond to randomized controlled trials. |
|
What types of patients were included* |
Six trials included patients with osteoarthritis of the knee, [6],[10],[11],[12],[13],[14], two with hip osteoarthritis [7], [9] and one of the trials included hip or knee osteoarthritis [8]. The mean age range of patients included in the trials was 47 to 64 years. The average age of women included in the trials ranged from 55% to 83%. |
|
What types of interventions were included* |
All trials administered oral diacerein, seven of them 50 mg twice daily [6],[7],[8],[9],[10],[11],[12] and in two trials this data could not be obtained (the researchers did not specify the data [13],[14]). All trials compared diacerein against placebo. |
|
What types of outcomes |
The systematic reviews grouped outcomes as follows:
|
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
Summary of findings
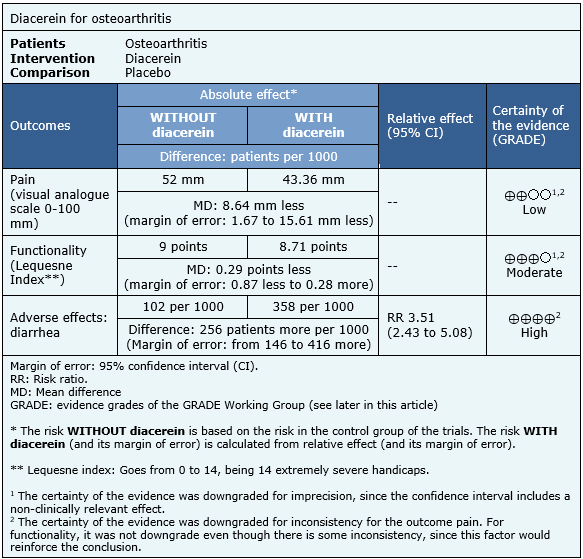
The information on the effects of diacerein on osteoarthritis is based on nine randomized trials. Six trials measured the outcome pain [6],[7],[8],[9],[11],[12], four trials measured the outcome functionality [7],[8],[9],[12] and seven trials measured the occurrence of diarrhea [6],[7],[8],[9],[10],[11],[12]. The summary of findings is as follows:
- Diacerein may produce a slight decrease in pain in patients with osteoarthritis, but the certainty of the evidence is low.
- Diacerein probably not produce improvement in function in patients with osteoarthritis. The certainty of the evidence is moderate.
- Diacerein frequently produces diarrhea. The certainty of the evidence is high.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
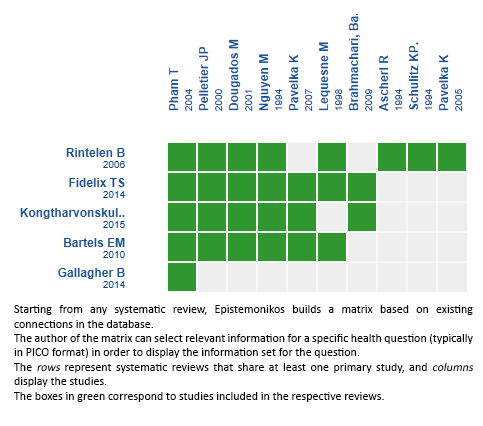
Follow the link to access the interactive version: Diacerein versus placebo or no treatment for osteoarthritis
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.

