Epistemonikos summaries
← vista completaPublished on January 18, 2018 | http://doi.org/10.5867/medwave.2018.01.7138
Is mytomicyn better than 5-fluorouracil as antimetabolite in trabeculectomy for glaucoma?
¿Es la mitomicina C superior al 5-fluorouracilo como antimetabolito en la trabeculectomía para el glaucoma?
Abstract
INTRODUCTION Trabeculectomy is considered the standard for glaucoma surgery. Postoperative scarring is one the factors associated with surgery failure. Different antimetabolites have been used in order to reduce this risk, particularly 5-fluorouracil and mitomycin C. Although both are considered effective, it is not clear if they are different in terms of success of trabeculectomy and adverse effects.
METHODS To answer this question we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS We identified four systematic reviews including 17 studies overall, of which 12 were randomized trials. We concluded mitomycin C might be more effective in reducing intraocular pressure and increasing qualified success compared to 5-fluorouracil. However, its use might be associated to a higher risk of complications.
Problem
According to the World Health Organization, glaucoma is the second cause of blindness worldwide. Among the known risk factors for the development of this disease, the intraocular pressure is the only one that can be modified. Trabeculectomy is the surgery of choice for patients with medically uncontrolled glaucoma.
Antimetabolites have been used as a strategy to improve the success of trabeculectomy, with the aim of reducing postsurgical scarring and thus favoring the aqueous humor outfow to the subconjuntival space. 5-fluorouracil and mitomycin C are considered effective, but it is not clear if there are differences in terms of success and risk of complications, such as bleb leak, cataract formation, ocular hypotonia and endophthalmitis.
Methods
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found four systematic reviews [1],[2],[3],[4] that included 17 primary studies reported in 19 references [5],[6],[7],[8],[9],[10],[11],[12],[13],[14],[15],[16], This table and the summary in general are based on 11 randomized trials, since the observational studies did not increase the certainty of the evidence or provide additional relevant information. |
|
What types of patients were included* |
Regarding the type of glaucoma, five trials included patients with more than one type of glaucoma [6],[8],[15],[21],[22], four trials included patients with open-angle glaucoma [10],[13],[19],[23]. In two trials, the type of glaucoma was not reported [9],[12]. Regarding the risk of trabeculectomy failure, four trials included patients considered to be as high risk [6],[8],[9],[13], five included patient with a low risk of failure [10],[15], [19],[21],[23] and one trial included both high and low risk patients [22]. In one trial, the risk of trabeculectomy failure was not reported [12]. |
|
What types of interventions were included* |
All trials compared mitomycin C against 5-fluorouracil [6],[8],[9],[10],[12],[13],[15],[19],[21],[22],[23]. The use of mitomycin was during the intraoperative period in all of the trials [6],[8],[9],[10],[12],[13], The use of 5-fluorouracil was during the intraoperative period in six trials [10],[12],[13],[15],[19],[21], of which in five a solution of 50 mg/ml was administered for 5 minutes [12],[13],[15],[19],[21] and a dose of 5 mg was administered in one trial [10]. In the remaining five trials, 5-fluorouracil was used postoperatively [6],[8],[9],[22],[23]; in three trials 10 doses of 5 mg were administered [6],[8],[9], and in two 7 doses of 5 mg were administered [22],[23]. |
|
What types of outcomes |
The trials measured multiple outcomes, which were grouped by the systematic reviews as follows:
|
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
Summary of Findings
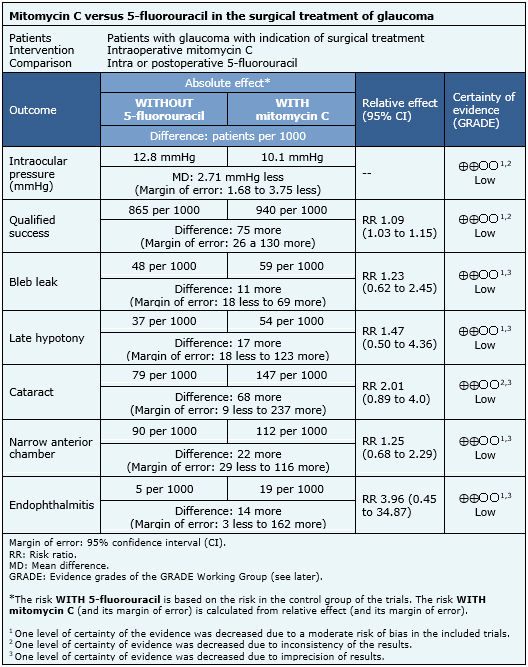
The information on the effects of mitomycin C compared to 5-fluorouracil for trabeculectomy is based on 11 randomized trials involving 770 eyes.
Seven trials reported mean intraocular pressure at the end of follow-up or at one year (386 eyes) [6],[8],[9],[10],[13],[21],[23], 10 trials evaluated qualified success (594 eyes) [6],[8],[9],[10],[13],[15],[19],[21],[22],[23], eight trials evaluated bleb leak (545 eyes) [6],[8],[9],[13],[15],[21],[22],[23], four trials evaluated late hypotony (211 eyes) [10],[13],[22],[23], three trials evaluated development of cataract (235 eyes) [6],[13],[21], five trials evaluated narrow anterior chamber (311 eyes) [8],[13],[15],[22],[23] and four trials evaluated endophthalmitis (315 eyes) [6],[9],[13],[21].
The summary of findings is as follows:
- Mitomycin C might be more effective in reducing intraocular pressure than 5-fluorouracil, but the certainty of the evidence is low.
- Mitomycin C might increase qualified success rate compared to 5-fluorouracil, but the certainty of the evidence is low.
- Mitomycin C might be associated to a higher incidence of bleb leak, but the certainty of the evidence is low.
- Mitomycin C might be associated to a higher incidence of late hypotony, but the certainty of the evidence is low.
- Mitomycin C might be associated to a higher incidence of cataract development, but the certainty of the evidence is low.
- Mitomycin C might be associated to a higher incidence of narrow anterior chamber, but the certainty of the evidence is low.
- Mitomycin C might be associated to a higher incidence of endophthalmitis, but the certainty of the evidence is low.
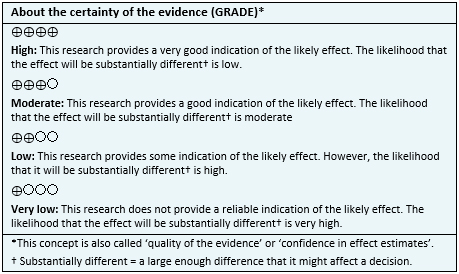
| Following the link to access the interactive version of this table (Interactive Summary of Findings- iSoF) |
Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
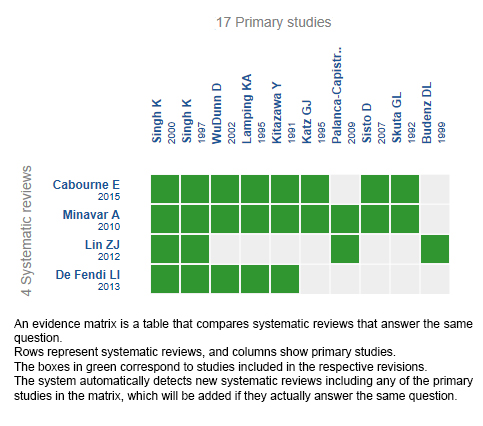
Follow the link to access the interactive version: 5-fluorouracil compared with mitomycin C in glaucoma
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.

