Epistemonikos summaries
← vista completaPublished on March 27, 2021 | http://doi.org/10.5867/medwave.2021.02.8140
Antibiotics for acute uncomplicated diverticulitis in hospitalized patients
Antibióticos para diverticulitis aguda no complicada en pacientes hospitalizados
Abstract
Introduction Acute diverticulitis is one of the complications of diverticular disease. Nowadays, there is a paradigm shift regarding the use of antibiotics to manage acute uncomplicated diverticulitis in hospitalized patients, with controversial information about it.
Methods A search was done in Epistemonikos, the most comprehensive health-related systematic review database, maintained by screening multiple information sources including MEDLINE/PubMed, EMBASE, Cochrane, among others. Data were extracted from the identified systematic reviews, data from primary studies were analyzed, which in this work considered only randomized clinical trials, a meta-analysis was done, and a summary table of results was created using GRADE methodology.
Results and conclusions Eleven systematic reviews were identified that included seven primary studies in total, of which two were randomized control trials. We concluded that the use of antibiotics in acute uncomplicated diverticulitis could slightly increase complications and result in a minor or no difference in the risk of recurrence and need for urgent surgery. However, the certainty of the evidence is low. Regarding hospital stay and readmission, it was not possible to evaluate the effect due to a low certainty of evidence.
Problem
Acute diverticulitis is a complication of diverticular disease in which inflammation of one or more diverticula occurs. It occurs in about 5% of individuals with diverticula[1], of which around 75% of cases correspond to acute uncomplicated diverticulitis, which refers to the presence of diverticular inflammation and the absence of complications such as intestinal perforation, abscesses, obstruction, or fistulas[2]. The clinical condition is composed of abdominal pain, changes in bowel habits, abdominal distention, nausea, fever, and pain on palpation of the left lower quadrant[3].
In cases of acute uncomplicated diverticulitis, hospital management is preferred when dealing with patients with poor oral tolerance, severe pain, comorbidities, those who are elderly, immunocompromised, or who failed with outpatient treatment[2]. In these cases the standard treatment consists of administering antibiotics and dietary restriction, and symptom control[3].
In recent years, the use of antibiotics as a cornerstone of treatment has been challenged, arguing that antibiotics would not change the clinical outcomes presented by patients. Furthermore, the fact that the antibiotics can lead to adverse effects or poor tolerance by patients must also be considered[4],[5].
Methods
A search in Epistemonikos, the largest health-related systematic review database, was done by filtering multiple sources of information, including MEDLINE/PubMed, EMBASE, Cochrane, among others. For the search, the keywords were “acute diverticulitis”, “colonic diverticulitis”, “diverticular disease”, “antibiotic”, “antibacterial” and “bactericide agent”. Along with this, a search was carried out in Google Scholar to evaluate the existence of gray literature.
Data were extracted from the identified systematic reviews that answered the clinical question posed. The data from the primary studies were examined, which in this study only corresponded to randomized clinical trials since they are considered the best source of evidence, excluding observational studies.
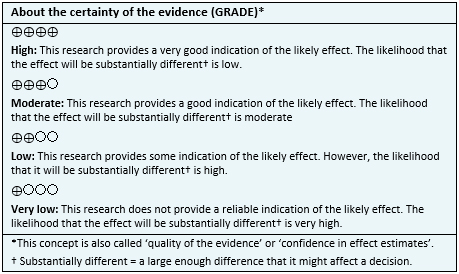
With this information, a structured summary called FRISBEE (Friendly Summaries of Body of Evidence using Epistemonikos) was created, by following a pre-established format, including key messages, a summary of the body of evidence (presented as a matrix of evidence in Epistemonikos), a meta-analysis of all studies when possible, a summary table of results using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) method, and a section on other considerations for decision-making.
|
Main messages
|
In relation to the body of evidence to answer these questions
|
What is the evidence?
|
We found 11 systematic reviews[6],[7],[8],[9],[10],[11],[12],[13],[14],[15],[16] which included seven primary studies that answer the clinical question, two of them being randomized trials[17],[18]. This table and the general summary are based on the latter trials since the observational studies did not increase the certainty of the existing evidence, nor did they provide additional relevant information. |
|
What type of patients were recruited in the studies? * |
All trials included patients with acute uncomplicated diverticulitis, clinically diagnosed using computed tomography. All patients included in the trials were older than 18 years old[17],[18]. The age range in one trial was between 48 and 64 years old[17], while in the other trial, the mean age of patients was 57 years old[18]. One trial recruited patients with a medical history of previous acute diverticulitis[18]. Patients diagnosed with or suspected of other diseases on computed tomography, such as colon cancer and inflammatory bowel disease, were excluded. |
|
What types of interventions were used in the studies? * |
In both trials, patients were managed as inpatients. One trial used the administration of intravenous antibiotics, amoxicillin plus clavulanic acid, for two days and then switched to an oral scheme for eight days. In allergic patients, ciprofloxacin was used in addition to metronidazole for ten days[17]. The other trial used the administration of intravenous antibiotics as intervention, cefuroxime or cefotaxime, in addition to metronidazole, carbapenems, or piperacillin-tazobactam. Then an oral scheme with ciprofloxacin or cefadroxil in addition to metronidazole. Antibiotic therapy lasted for at least seven days[18]. As a comparison, both trials used a symptomatic treatment without antibiotics, with intravenous fluids and supportive measures[17] or only fluids[18]. |
|
What kind of outcomes were measured? |
The outcomes measured were the following:
|
* Information on primary studies was collected from identified systematic reviews, not directly from studies unless otherwise specified.
Summary of the results
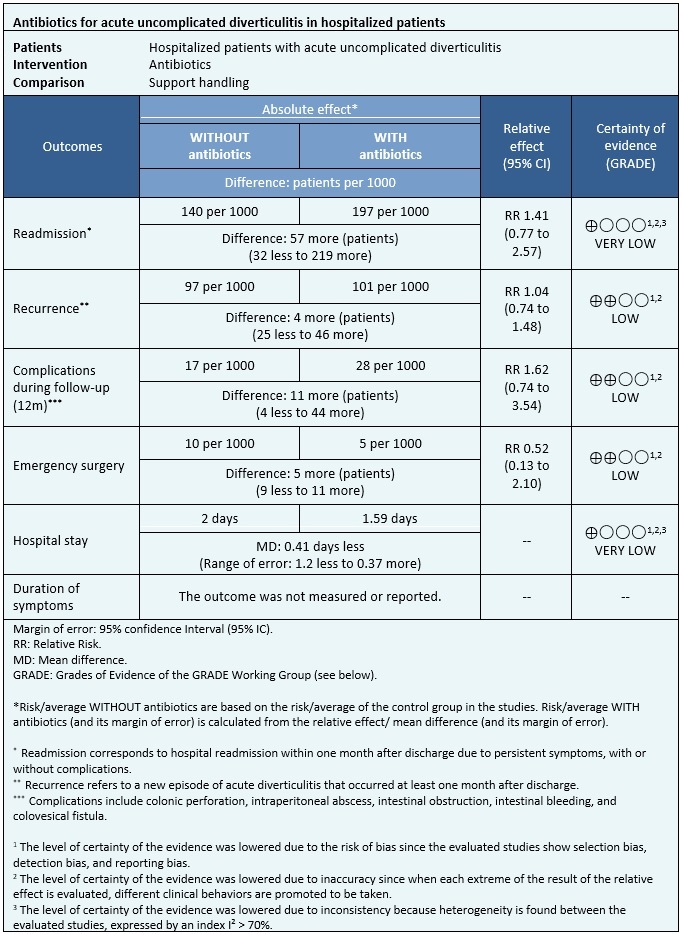
Information on the effects of antibiotic use for acute uncomplicated diverticulitis in hospitalized patients is based on two randomized trials that included 1151 patients.
In both trials, the outcomes of readmission, length of hospital stay, complications during follow-up, need for emergency surgery (1151 patients), and recurrence (1110 patients) were measured[17],[18].
The summary of the results is as follows:
- It is not possible to clearly establish whether the use of antibiotics leads to increased readmission as the certainty of the evidence was assessed as very low.
- The use of antibiotics could make little or no difference in the risk of recurrence of acute diverticulitis (low certainty of the evidence).
- The use of antibiotics makes little or no difference in the risk of developing complications during the follow-up of patients with acute uncomplicated diverticulitis (low certainty of the evidence).
- The use of antibiotics could make little or no difference in the risk of needing emergency surgery in patients with acute uncomplicated diverticulitis (low certainty of the evidence).
- It is not possible to clearly establish whether the use of antibiotics decreases the duration of the hospital stay since the certainty of the evidence was evaluated as very low.
- No trials evaluating the duration of symptoms were found.
|
Follow the link to access the interactive version of this table (Interactive Summary of Findings - iSoF) |
Other considerations for decision making
|
Whom does this evidence apply to? |
|
|
In relation to the outcomes included in this summary |
|
|
Harm/benefit balance and certainty of the evidence |
|
|
Resource considerations |
|
|
What do patients and their caregivers think? |
|
|
Differences between this summary and other sources |
|
|
Could this information change in the future? |
|
How did we do this summary?
Using automated and collaborative methods, we collected all the relevant evidence for the question of interest which was presented in an evidence matrix.
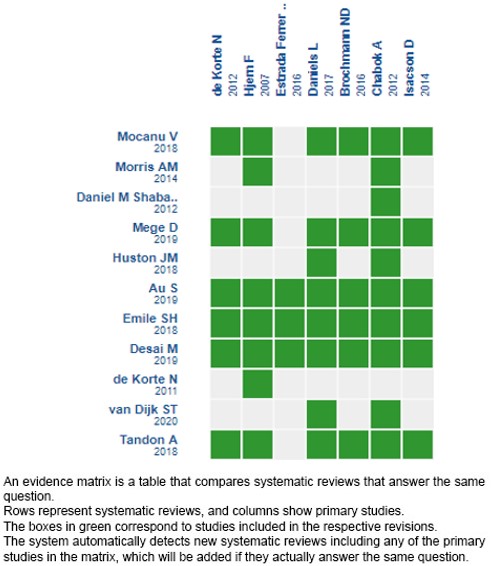
Please, follow the link to access the interactive version: Antibióticos versus manejo sintomático para el tratamiento de diverticulitis aguda no complicada en pacientes hospitalizados.
Notes
If new systematic reviews on this topic are published after the publication of this summary, a notice of “new evidence” will be displayed at the top of the matrix. Although the project contemplates the periodic updating of these summaries, users are invited to comment on the Medwave website or contact the authors by email if they believe that there is evidence that motivates an earlier update.
After creating an account on Epistemonikos, when saving the matrices, you will receive automatic notifications every time there is new evidence that may potentially answer this question.
This paper is part of the Epistemonikos Evidence Synthesis Project. It is prepared with a pre-established methodology, following rigorous methodological standards and an internal peer review process. Each of these papers corresponds to a summary, called FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the set of evidence of a specific question in a friendly format for clinical professionals. Its main resources are based on the Epistemonikos evidence matrix and the analysis of results using the GRADE methodology. Further details of the methods to make this FRISBEE are described here: (http://dx.doi.org/10.5867/medwave.2014.06.5997)
The Epistemonikos Foundation is an organization that seeks to bring information to those who make decisions in health through the use of technologies. Its main development is the Epistemonikos database.(www.epistemonikos.org).
Contribution roles
CAQ: conceptualization, methodology, software, validation, formal analysis, research, resources, data management, manuscript, manuscript revision, visualization, project administration and acquisition of funds. LTB: conceptualization, methodology, validation, formal analysis, research, resources, data management, manuscript, visualization, project administration and acquisition of funds. GGB: conceptualization, methodology, validation, formal analysis, research, resources, data management, manuscript, visualization and acquisition of funds. AZC: conceptualization, validation, manuscript revision, visualization, monitoring, project administration and acquisition of funds.
Acknowledgments
We would like to thank doctors Francisca Verdugo and Camila Avila for introducing and instructing the work team in the FRISBEE method
Competing interests
The authors declare that they have no conflicts of interest with the subject of this manuscript.
Ethical statement
Given that this research is a study on secondary sources of information, it does not need approval by an ethics committee.
Funding
School of Medicine, Universidad Finis Terrae, Santiago, Chile.
Data repository declaration
The data are available upon request and evaluation of the rationale by the authors.

