Epistemonikos summaries
← vista completaPublished on December 21, 2021 | http://doi.org/10.5867/medwave.2021.11.8155
Perspectives of patients and consumers on the use of generic medicines
Cuáles son las perspectivas de los pacientes o consumidores sobre el uso de medicamentos genéricos
Abstract
INTRODUCTION Access to medicines constitutes a public health challenge worldwide. Promoting utilization of generic medicines is one of the strategies that has been proposed to optimize pharmaceutical spending and thus allow greater coverage. However, its use is not yet widespread enough. This study seeks to explore the perspectives and acceptability to the use of generic medicines from patients and consumers.
METHODS We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a evidence synthesis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS We identified four systematic reviews that together include 47 primary studies, of which 1 corresponds to a randomized trial. A low rate of patients or consumers has a negative perception regarding generic medicines, including dimensions such as risk, quality, safety, risk of adverse effects, among others.
Problem
Access to medicines has become a challenge for health systems worldwide, as it represents a significant proportion of out-of-pocket household expenses. Along these lines, the World Health Organization has encouraged states to generate policies pointing to the rational use of medicines and to the increase of generic medicines utilization [1]. The use of generic medicines would enable savings in out-of-pocket consumer spending and result in a more efficient pharmaceutical spending by insurers and health systems, becoming a cost containment strategy [2].
However, although the generic substitution (interchangeability of a brand-name medicine for a generic medicine) is allowed in multiple countries, this practice has not been widely adopted. Potentially, negative perceptions over the quality and safety of generic medicines across different actors could be an explanatory factor for this phenomenon [3]. In this context, it is important to analyze the scientific evidence available in this area, thus the objective of this article is to explore the perception that consumers or patients have regarding generic medicines.
For the purposes of this summary, patients will be understood as the people who attend a healthcare center for any health condition and consumers as those who buy a drug from a pharmacy or may need to buy a drug in the future (general population).
Methods
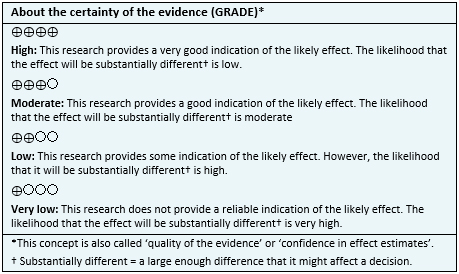
We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We identified four systematic reviews [3], [4], [5], [6] including 47 studies overall [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], of which one was a randomized trial [38]. The table and summary are based on the totality of the studies since observational studies provide relevant information for this type of question. It is also worth mentioning that 31 studies conducted surveys [7], [8], [9], [10], [11], [15], [16], [18], [19], [20], [22], [24], [25], [26], [27], [29], [30], [31], [34], [35], [36], [37], [39], [41], [43], [44], [47], [48], [49], [50], [52], 10 interviews [12], [13], [14], [17], [23], [38], [42], [45], [51], [53] and 6 focus groups [21], [28], [32], [33], [40], [46]. |
|
What kind of population included the studies |
All the studies included patients or consumers older than 18 years of age except for two studies which included consumers over 15 years [18], [27]. According to the World Bank’s country classification by income for the period 2018-2019 [54], ten studies (21.3%) were conducted in upper-middle income countries [8], [19], [23], [25], [27], [32], [33], [42], [47], [48] and 37 (78.7%) in high-income countries [7], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [20], [21], [22], [24], [26], [28], [29], [30], [31], [34], [35], [36], [37], [38], [39], [40], [41], [43], [44], [45], [46], [49], [50], [51], [52], [53]. Twenty-six studies focused in people with a specific condition [9], [13], [14], [17], [21], [22], [26], [28], [29], [31], [34], [36], [37], [38], [40], [41], [43], [44], [45], [46], [47], [49], [50], [51], [52], [53], and the remaining 21 included consumers in general [7], [8], [10], [11], [12], [15], [16], [18], [19], [20], [23], [24], [25], [27], [30], [32], [33], [35], [39], [42], [48]. Within the latter, 10 studies were sampled from the general population [7], [8], [15], [16], [18], [19], [20], [23], [27], [35]. |
|
What types of outcomes were measured |
The studies evaluated multiple outcomes, which were grouped by systematic reviews as negative perception of:
|
* Information about primary studies was primarily extracted from the systematic reviews identified and was completed with data extracted directly from primary studies.
Summary of findings
The information on the perception of the use of generic medicines is based on the information reported by 46 observational studies [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53] and one randomized trial [38], varying the total number of the sample, according to each outcome measured.
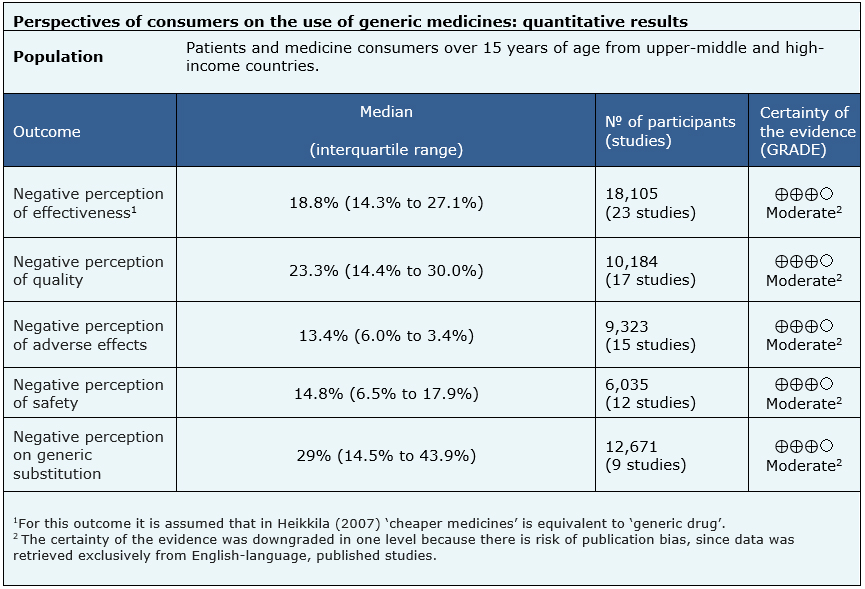
The quantitative results of the negative perception of effectiveness are based on 23 studies (18 105 participants) [7], [8], [10], [11], [15], [17], [18], [19], [24], [25], [27], [29], [30], [31], [34], [35], [36], [39], [47], [48], [49 ], [51], [52], those of the negative perception of quality are based on 17 studies (10 184 participants) [7], [8], [9], [17], [18], [19], [23 ], [25], [26], [29], [30], [31], [35], [39], [45], [47], [52], the negative perception of adverse effects are based on 15 studies (9 323 participants) [15], [18], [24], [25], [29], [39], [43], [44], [45], [47], [48], [49], [50], [51], [52], the negative perception of safety on 12 studies (6 035 participants) [10], [11], [17], [19], [29], [30], [34], [35], [36], [39], [51], [52] and the results of the negative perception on generic substitution on 9 studies (12 671 participants) [10], [16] , [20], [22], [37], [41], [43], [48], [50].
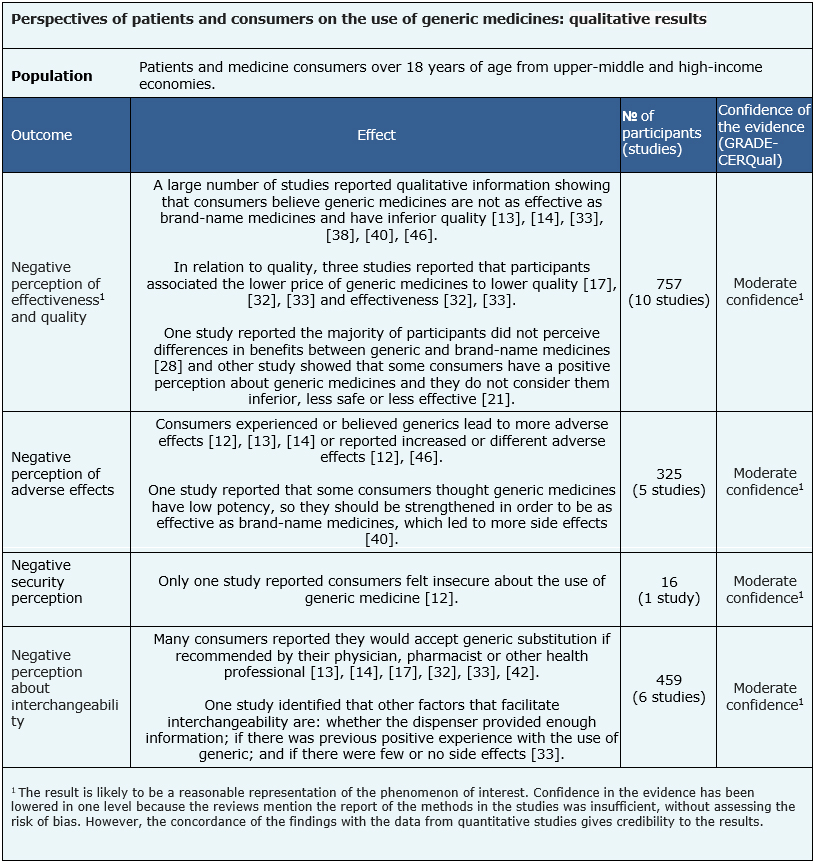
The qualitative results of the negative perception of effectiveness and quality are based on 10 studies (757 participants) [13], [14], [17], [21], [28], [32], [33], [38], [40], [46], those of the negative perception of adverse effects are based on 5 studies (325 participants) [12], [13], [14], [40], [46], the negative perception of safety on 1 study (16 participants) [12] and those of the negative perception on generic substitution on 6 studies (459 participants) [13], [14], [17], [32], [33], [42].
The summary of findings is as follows:
- A low proportion of consumers or patients have a negative perception regarding the effectiveness of generic medicines (moderate certainty of the evidence).
- A low proportion of consumers or patients have a negative perception regarding the quality of generic medicines (moderate certainty of the evidence).
- A low proportion of consumers or patients perceive generic medicines increase the risk of adverse effects (moderate certainty of the evidence).
- A low proportion of consumers or patients have a negative perception of the safety of generic medicines (moderate certainty of the evidence).
- A low proportion of consumers or patients have a negative perception about generic substitution (moderate certainty of the evidence).

Other considerations for decision-making
|
Contex analysis |
|
| To whom this evidence does and does not apply |
|
| Certainty and confidence of the evidence |
|
| Resource considerations |
|
| Differences between this summary and others sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
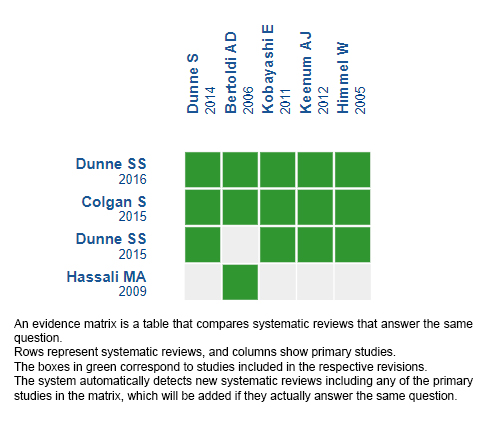
Follow the link to access the interactive version: Perspectives of patients and consumers on the use of generic medicines.
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.

