Epistemonikos summaries
← vista completaPublished on May 4, 2021 | http://doi.org/10.5867/medwave.2021.04.8166
Aflibercept compared with dexamethasone in diabetic macular edema
Aflibercept comparado con dexametasona en edema macular diabético
Abstract
INTRODUCTION Diabetic macular edema is a frequent pathology that causes gradual deterioration of visual acuity, which does not have a standardized treatment. The anti-vascular endothelial growth factor (anti-VEGF) drugs and corticosteroids are widely used, especially aflibercept and dexamethasone, respectively, but it is unclear which one is best.
METHODS We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS No evidence that compared the interventions directly in the population of interest was found, so systematic reviews that provide an estimate of the effect indirectly using network meta-analysis were selected. We identified two systematic reviews that together included four primary studies, all randomized trials. We concluded that we are uncertain whether aflibercept compared to dexamethasone improves visual acuity or is safer, as the certainty of the evidence has been assessed as very low.
Problem
Diabetic macular edema is a manifestation of diabetic retinopathy, one of the most frequent eye diseases worldwide. The usual presentation of this condition is gradual deterioration of visual acuity, which without treatment is usually associated with a poor prognosis. For many years, laser therapy was used to reduce symptoms, but its results and multiple complications increased the urge to find alternative therapies. Nowadays, the most commonly used treatment by specialists is periodic intravitreal injection of anti-vascular endothelial growth factor drugs (anti-VEGF), among which aflibercept stands out, mainly due to its longer half-life, and corticosteroids, more specifically dexamethasone. Despite the above, there is no clear superiority between them, so it is important to summarize the evidence that allows us to compare both treatments..
Methods
We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
No evidence that compares the interventions directly in the population of interest was found, so systematic reviews that provide an estimate of the effect indirectly using network meta-analysis were selected. We found two systematic reviews [1], [2], which included four primary studies [3], [4], [5], [6], all randomized trials. This summary is based on the latter, since they evaluate the intervention in the population of interest, but against a different comparison. |
|
What types of patients were included* |
All trials included patients with the diagnosis of diabetic macular edema. None of the reviews reported if all the included patients had central involvement. |
|
What types of interventions were included* |
Two trials evaluated aflibercept [5], [6]: one compared it against other medications [4] and the other trial compared it against placebo [6]. Two trials evaluated dexamethasone [3], [5]: one evaluated the addition of dexamethasone to laser treatment [3] and the other compared it against another drug [5]. |
|
What types of outcomes |
The trials reported multiple outcomes, which were grouped by systematic reviews as follows:
|
*Information about primary studies is not extracted directly from primary studies but from identified systematic reviews, unless otherwise stated.
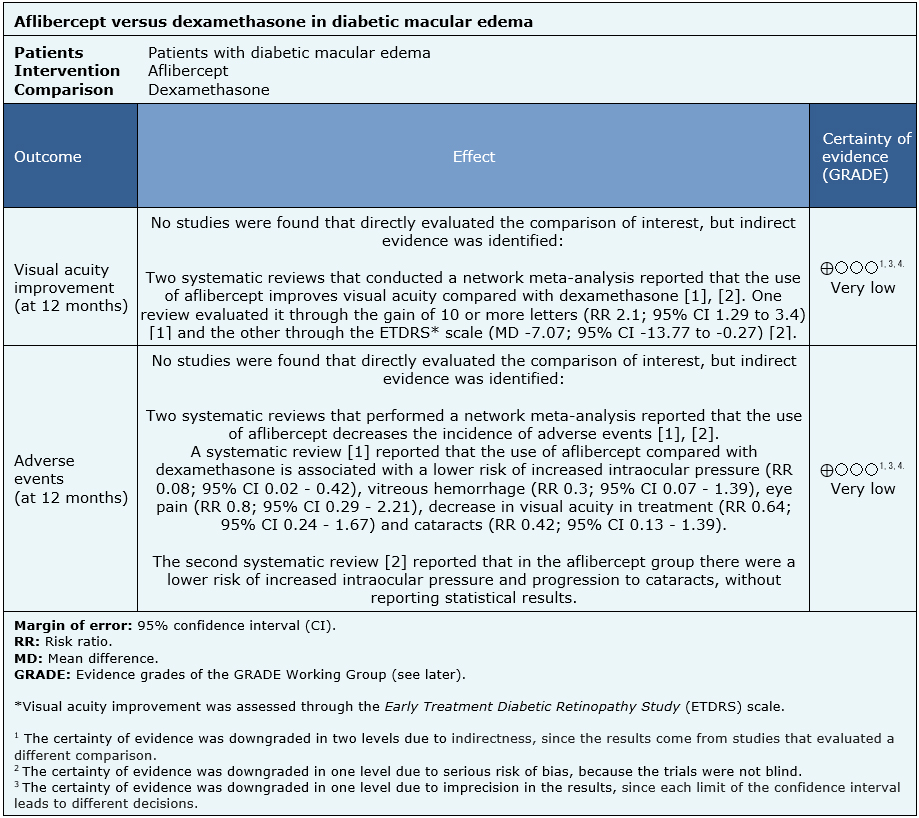
Summary of findings
No direct evidence that compares aflibercept with dexamethasone in diabetic macular edema was found. Information of the effects of aflibercept compared to dexamethasone in diabetic macular edema is based on two systematic reviews that performed an indirect comparison using network meta-analyses [1], [2].
Since no studies were identified evaluating the question directly, the results in the summary of findings table are based on punctual estimators from each of the systematic reviews. All reviews [1], [2] assessed visual acuity outcomes and adverse events.
The summary of findings is as follows:
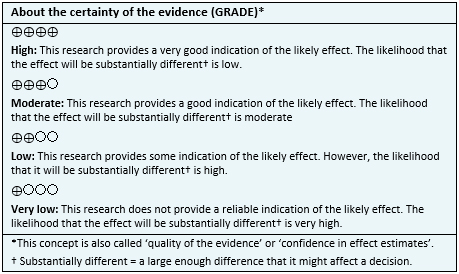
- We are uncertain whether aflibercept compared to dexamethasone improves visual acuity as the certainty of the evidence has been assessed as very low.
- We are uncertain whether aflibercept compared to dexamethasone reduces adverse events as the certainty of the evidence has been assessed as very low.
| Follow the link to access the interactive version of this table (Interactive Summary of Findings – iSoF) |
Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
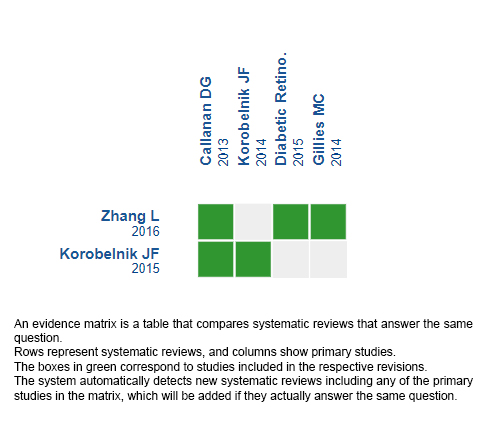
Follow the link to access the interactive version: Aflibercept versus dexamethasone in diabetic macular edema.
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.

