Epistemonikos summaries
← vista completaPublished on June 23, 2021 | http://doi.org/10.5867/medwave.2021.05.8213
Autologous serum compared to artificial tear drops for dry eye disease
Suero autólogo comparado con lágrimas artificiales para ojo seco
Abstract
Introduction Dry eye is one of the most common ocular surface disorders. Although artificial tear drops therapy is the most widely used treatment, it has recently been suggested that autologous serum could be a beneficial alternative treatment for this disorder, but its use is controversial.
Methods We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE/PubMed, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
Results and conclusions We identified six systematic reviews, including seven primary studies overall, of which all were randomized trials. We concluded that autologous serum treatment might not lead to adverse effects compared to artificial teardrops, but the certainty of the evidence is low. On the other hand, we are uncertain whether autologous serum therapy improves the quality of life, severity of the pathology, pain or the corneal epitheliopathy grade compared to artificial tear drops as the certainty of the evidence has been assessed as very low.
Problem
Dry eye affects hundreds of millions of people worldwide. It is a chronic multifactorial disease of the ocular surface characterized by a loss of tear film homeostasis. Several factors play an etiological role in this condition. These factors are tear film instability and hyperosmolarity, ocular surface inflammation, damage and neurosensory abnormalities [1]. Common symptoms are irritation or burning sensation, foreign body sensation, visual disturbances, blurred vision, photophobia and pain.
Artificial tears are the most commonly used treatment. However, artificial tears differ in their components from physiological tears. For this reason, other alternatives have been proposed that consider pathophysiology elements in their composition. One alternative is autologous serum.
Autologous serum contains several growth factors involved in the epithelial migration process, necessary for ocular surface repair and maintenance of tear stability. These factors are not present in artificial tears. Some of these factors are epithelial growth factor, nerve growth factor, fibronectin and vitamin A. It has also been shown that the use of autologous serum would inhibit the release of inflammatory cytokines. This process would generate an environment that enables tear film stabilization, promoting epithelial migration and fibroblastic activation necessary for corneal repair [1]. However, its use is controversial.
Methods
We searched Epistemonikos, the largest database of systematic reviews in health, which is maintained by multiple sources of information. These sources include MEDLINE/PubMed, EMBASE, and Cochrane, among others. We extracted and analyzed data from the identified reviews and primary studies. We generated a structured summary called FRISBEE (Friendly Summaries of the Body of Evidence using Epistemonikos), following a pre-established format with this information. This format includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analyses of all studies when possible, a summary table of results using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) method, and a section on other decision-making considerations.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found six systematic reviews [2],[3],[4],[5],[6],[7], which included seven primary studies reported in eight references [8],[9],[10],[11],[12],[13],[14],[15] of which, all are randomized trials. |
|
What type of patients did the studies include? * |
All trials included patients with dry eye [8],[9],[10],[11],[12],[13],[14]. The patients average age included in the trials was 46 years. Three trials [8],[9],[14] included patients with severe dry eye defined as Schirmer's test score less than five millimeters and tear film breakup time less than five seconds. Two trials [8],[9] included patients with fluorescein staining scores greater than or equal to one on the Oxford scale. Only one trial included patients with dry eye due to Sjögren's syndrome [11]. One trial included only male patients with dry eye following surgery with the laser-assisted in situ keratomileusis technique [12]. Two trials did not define diagnostic criteria for dry eye [10],[13]. Regarding exclusion criteria, one trial excluded patients with uncontrolled cerebrovascular or cardiovascular disease, history of refractive surgery, lactating or pregnant women [8]. Two trials excluded patients with any other ocular pathology, severe anemia, previous use of autologous serum or use of drops for other indications [8],[9]. In addition, two trials excluded patients with a history of lacrimal occlusion or the use of contact lens [8],[14]. The remaining trials reported no exclusion criteria [10],[11],[12],[13]. |
|
What type of interventions did the studies include? * |
All trials compared the use of autologous serum versus artificial tears [8],[9],[10],[11],[12],[13],[14]. Regarding differences in autologous serum concentration, five trials used 20% autologous serum [8],[9],[10],[12],[14], one trial used 50% autologous serum [11] and one trial used 40% autologous serum [13]. Regarding the frequency of autologous serum administration, two trials used autologous serum four times daily [8],[9]. Two trials used autologous serum five times daily [11],[12]. One trial used autologous serum six times daily [10]. The remaining trials did not report the frequency of autologous serum use [13],[14]. |
|
What type of outcomes did they measure |
The trials reported multiple outcomes, which were grouped by the systematic reviews as follows:
The average follow-up of the trials was three months, with a range between two weeks and 12 months. |
*Information on primary studies is extracted from the identified systematic reviews, not directly from the studies unless otherwise specified.
Summary of results
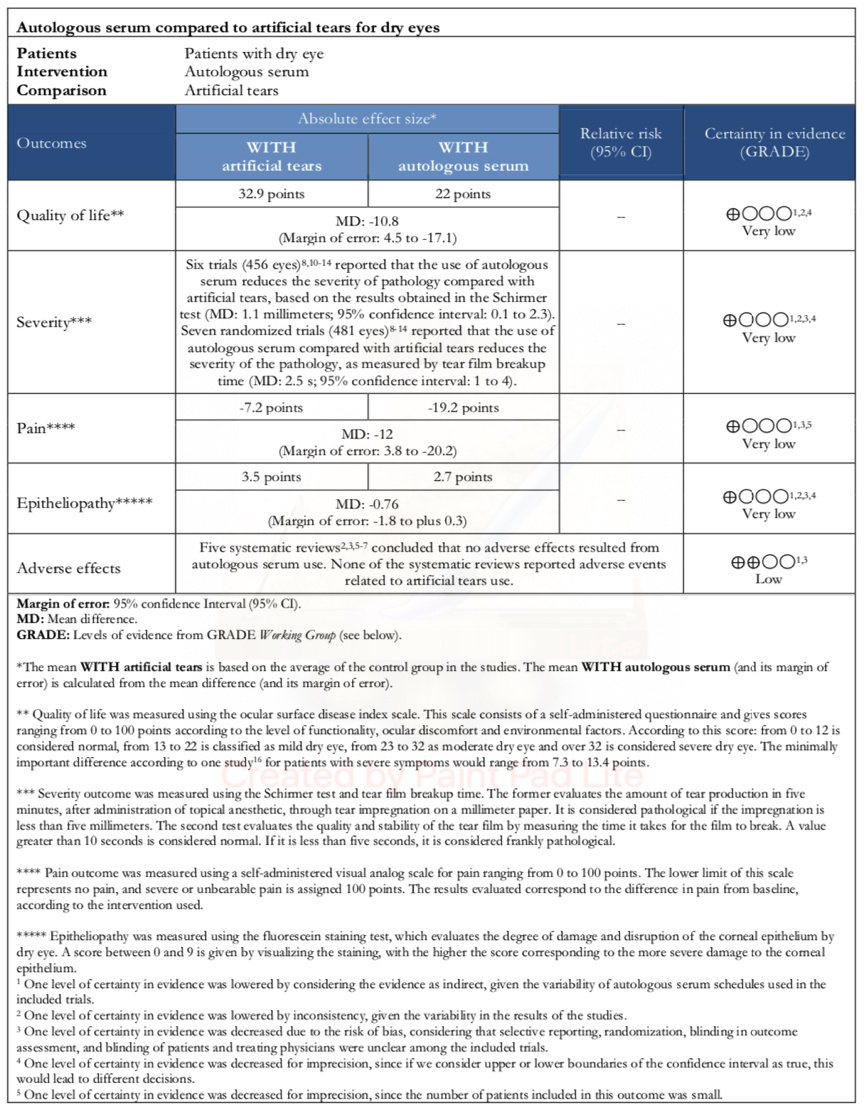
Information on the effects of using autologous serum compared with artificial tears for dry eye is based on seven randomized trials [8],[9],[10],[11],[12],[13],[14] involving 271 patients.
Four trials measured quality-of-life (160 eyes) [8],[9],[11],[13]. All trials measured dry eye severity by tear film breakup time test (481 eyes) [8],[9],[10],[11],[12],[13],[14]. Six trials assessed dry eye severity using the Schirmer test (456 eyes) [8],[9],[10],[11],[12],[13],[14]. Four trials measured corneal epitheliopathy (360 eyes) [8],[10],[12],[14], and only one trial assessed pain (20 eyes) [14].
All trials evaluated adverse effects associated with the use of autologous serum [8],[9],[10],[11],[12],[13],[14]. However, no review allowed the extraction of data in a way that could be incorporated into a meta-analysis. Thus, the information on this outcome is presented as a narrative synthesis.
The summary of the results is as follows:
- It is not possible to establish whether autologous serum improves the quality of life because the certainty of the existing evidence has been assessed as very low.
- It is not possible to establish whether autologous serum decreases the severity of the condition because the certainty of the existing evidence has been assessed as very low.
- It is not possible to establish whether the use of autologous serum decreases pain because the certainty of the existing evidence has been assessed as very low.
- It is not possible to establish whether autologous serum improves the degree of corneal epitheliopathy because the certainty of the existing evidence has been assessed as very low.
The use of autologous serum may have no adverse effects associated with its use (low certainty in evidence).
Follow the link to access the interactive version of this table (Interactive Summary of Findings - iSoF)
Other considerations for decision-making
|
To whom this evidence applies and to whom it does not apply. |
|
|
About the outcomes included in this summary |
|
|
Harm/benefit balance and certainty in evidence |
|
|
Costs |
|
What do patients and their caregivers think |
|
|
Differences between this summary and other sources |
|
|
Could this information change in the future? |
|
How we conducted this summary
We collected all the relevant evidence for this question and presented it in an evidence matrix using automated and collaborative methods.
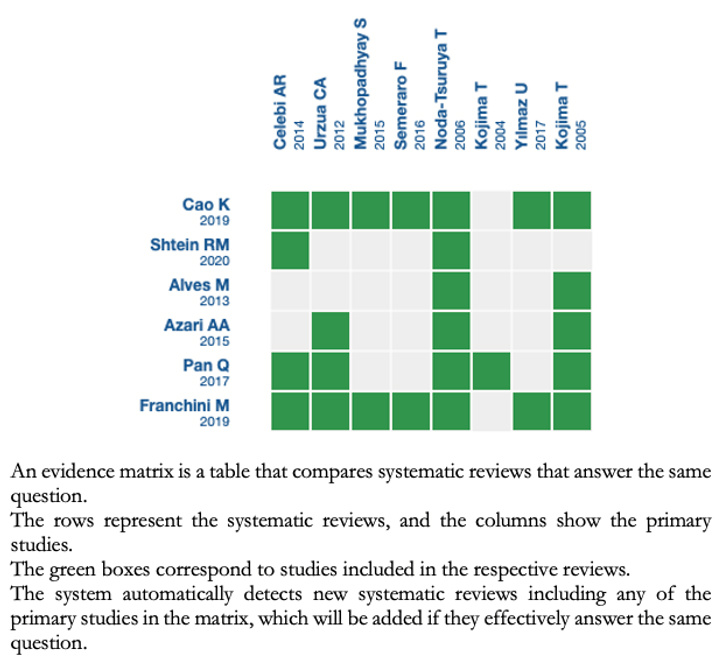
Follow the link to access the interactive version: Autologous serum compared to artificial tears for dry eye.
Notes
If new systematic reviews on this topic are published after the publication of this abstract, a "new evidence" notification will be displayed at the top of the matrix. While the project provides regular updates of these abstracts, users are invited to comment on the Medwave website or contact the authors by e-mail if they believe evidence warrants an earlier update.
After creating an Epistemonikos account, by saving the matrices, you will receive automatic notifications whenever there is new evidence that potentially answers this question.
This article is part of the Epistemonikos evidence synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and an internal peer review process. Each of these articles corresponds to a summary, called FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence of a specific question, in a friendly manner for physicians. The main resources are based on the Epistemonikos evidence matrix and the analysis of the result is based on the GRADE methodology. Further details of this FRISBEE elaboration method are described here.
The Epistemonikos Foundation is an organization that seeks to bring information closer to health decision-makers through the use of technologies. Its main source is the Epistemonikos database (www.epistemonikos.org).
Authorship contributions
FMY: methodology, validation, formal analysis, research, resources, data processing, writing, visualization and fund acquisition. GO: conceptualization, methodology, validation, formal analysis, research, resources, data processing and writing. CA: conceptualization, research, resourcing, writing, oversight, administration and fund adquisition.
Competing interest
The authors declare no competing interest.
Ethics
This study did not have an ethics committee evaluation, given the use of secondary data sources.
Funding
There were no external sources of funding for this study.
Language of submission
Spanish

