Analysis
← vista completaPublished on April 26, 2022 | http://doi.org/10.5867/medwave.2022.03.002526
Modeling of hantavirus cardiopulmonary syndrome
Modelamiento del síndrome cardiopulmonar por hantavirus
Abstract
Introduction Hantavirus cardiopulmonary syndrome is an infection caused by rodents of the Bunyanvirales family towards humans. This disease in Chile is considered endemic, which has a high fatality rate. At present, some studies show the contagion between people of the Andes virus, whose locality is concentrated in Argentina and Chile.
Objectives Analyze the possibility of hantavirus transmission between humans using an SEIR-type mathematical model.
Methods An SEIR (Susceptible, Exposed, Infectious and Recovered) mathematical model to express the dynamics of hantavirus disease is proposed, including the possibility of human-to-human transmission and the perception of risk.
Results The peak of human-to-human contagion decreases by about 25% after increasing people’s perception of risk by reducing the rate of resistance to changeand increasing the speed of people’s reaction.
Conclusions It is urgent to review risk communication strategies and prevention measures in the face of this possibility of massive human-tohuman infections, in addition to strengthening research and planning the development of a vaccine to protect populations exposed to this disease with a high fatality rate.
|
Main messages
|
Introduction
Hantavirus cardiopulmonary syndrome is an infection caused by rodents of the Bunyavirales order [1]. The Sin Nombre orthohan-tavirus and Andes virus are the main agents infecting humans within this order. Peromyscus maniculatus (deer mouse) and Oligoryzomys (long- tailed mouse) rodents are the natural reservoirs of hantavirus [1],[2],[3]. The transmission from rodents to humans is through inhalation of viral particles of urine, feces, or saliva [4],[5]. Although isolated, there are reported cases of transmission via bites.
In Chile, Hantavirus is an endemic disease [4] that produced 91, 33, 70, and 30 cases from 2017 to 2020, respectively [6]. Although its incidence is not high compared to other diseases, it has a fatality rate between 30 and 60% [7].
Person- to- person transmission of the Andes virus has been found in Argentina and Chile. In Argentina, the first confirmed case occurred in 1996, and in 2014 three other cases were reported [8]. In Chile in 2019, a possible person- to- person transmission was recorded from one person (index case) to her children, including a newborn, and to her daughter’s caregiver [4]. In addition, the virus was detected in breast milk, so trans-mission by this route cannot be ruled out. In Argentina, a study conducted between November 2018 and February 2019 recorded 34 confirmed person- to- person transmission cases in the province of Chubut [9]. The investigators found that patients with a high viral load and liver injury were more likely to spread the infection than other patients.
Mathematical models have contributed to understanding the dynamics of hantavirus transmission among rodents [10],[11],[12],[13],[14],[15],[16]. Some studies have modeled the demographic effect and associ-ated environmental variables [13],[14],[15],[16], and others have analyzed the rodent- to- rodent [10],[11],[12] and rodent-to-human transmission [17],[18]. However, there is a paucity of models incorporat-ing person- to- person hantavirus transmission – which is the main novelty of this work.
For rodent- to- human transmission dynamics of hantavirus, the studies mentioned above work with the premise of four infec-tion states: susceptible (S), exposed (E), infected (I), and recov-ered (R), commonly denoted by SEIR [19],[20],[21]. Infection in humans presents an incubation period of one to six weeks [6], then the host becomes symptomatic and may later acquire an immunity or die. Therefore, its disease evolution can be extrap-olated through the SEIR model.
A factor determining disease control is people’s risk perception [22],[23],[24],[25]. This aspect is supported by the decrease in hantavirus disease during years of a strong prevention publicity campaign in Chile [4],[26],[27].
This paper presents the dynamics of hantavirus disease using a mathematical SEIR model [19],[20],[21], which incorporates person- to- person transmission of the Andes virus.
Methods
Mathematical model
We based the dynamics of human- associated disease (h) on a SEIR model, which includes susceptible (S), exposed (E), infected (I), and recovered (R) states. An infected individual begins an incubation period (E) of one and six weeks and sub-sequently presents a symptomatic period (I) where the disease can be transmitted to other humans. Finally, the individual acquires immunity (R) or dies.
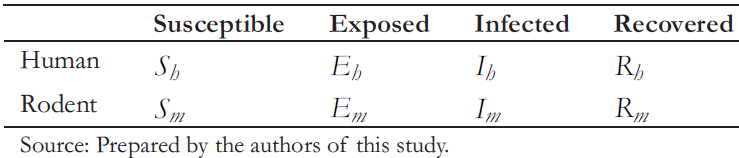
We also analyzed the rodent- to- rodent transmission (m) through the SEIR model. The transmission process is similar to humans but with different infection periods. Thus, to distin-guish the SIER states associated with the disease between humans and rodents, the subscript (h) is used for humans and (m) for rodents (Table 1).
 Full size
Full size A predetermining factor of rodent- to- human transmission is the risk perception (P) [28], which refers to people’s subjective judgments about adverse outcomes of disease, accidents, or death. The risk perception has a cognitive (i.e., what they know about hantavirus) and an emotional component (i.e., what they feel about hantavirus). Its assessment is essential to create prac-tical public health interventions and improve risk communica-tion since it informs about their perceived dangers and coping strategies. By taking the appropriate measures and generating an effective risk communication campaign, the probability of contagion can be significantly reduced. The risk perception (P) depends on a wide variety of factors, including resistance to change (λ1) and speed of reacting (λ2) to positive cases.
The model involving hantavirus dynamics between humans and rodents is given by the system of differential equations in.
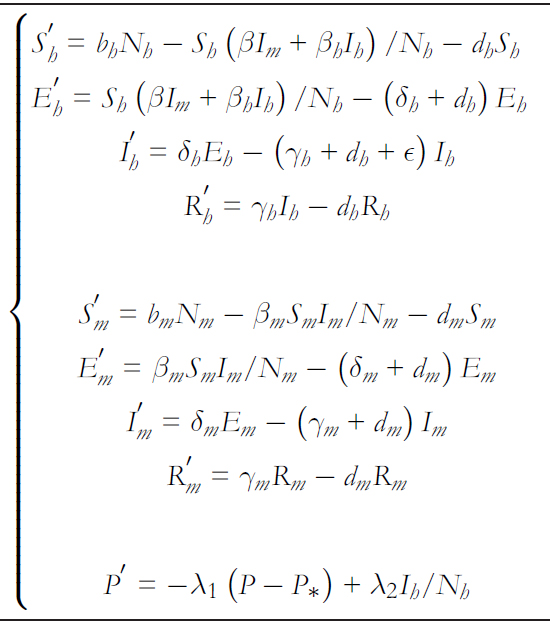
In this model, βh, βm y β corresponds to person- to- person, rodent- to rodent, and rodent- to- human contagion rates, respec-tively. Regarding person- to- person transmission rates ( βh and β ), these are dependent on the risk perception, with βh = βh∗ (P∗/P ) and β = β∗ (P∗/P) where βh∗ , β∗ y P∗ correspond to the average person- to- person and rodent- to- human transmission rates and the average quantified risk perception, respectively. Then, Nh and Nm denote the human and rodent populations, respectively. It is important to note that the rodent- to- human transmission βShIm/Nm is affected by the number of rodents er human ) K = Nm/Nh [29],[30],[31]. Thus, it finally remains βSh Im/Nm K = βShIm/Nh.
The transition rates from exposure (E) to infectious (I) states and from infectious to recovered (R) are given by δ and γ, respectively. Thus δ−1 and γ−1 determine the average time they remain in their incubated and infectious condition, respectively. The subscripts h and m differentiate the rates associated with humans and rodents, respectively. Birth and non disease mortality ates are expressed by bx and dx with x ∈ {h,m} espectively. THe disease lethality rate is given by ∈.
In mathematical epidemiology, an essential value to determine threshold conditions is the basic reproductive number R0 [32],[33], which is defined as "the expected number of secondary cases produced by a first infectious agent in a susceptible pop-ulation" [32], which establishes that if R0 >1 the disease spreads, and if R0 <1 it will not spread. In a local outbreak o the Andes virus in Argentina [9], the estimated reproductive number of transmissions between humans was 2.12 before announcing isolation measures for asymptomatic patients and self- quarantine for high- risk patients. Once control measures were applied, this estimate decreased to 0.96 [9]. From system (1), using the following generation method, the basic reproduc-tive number in the person- to- person transmission is given by
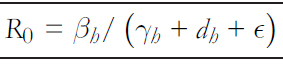
Background
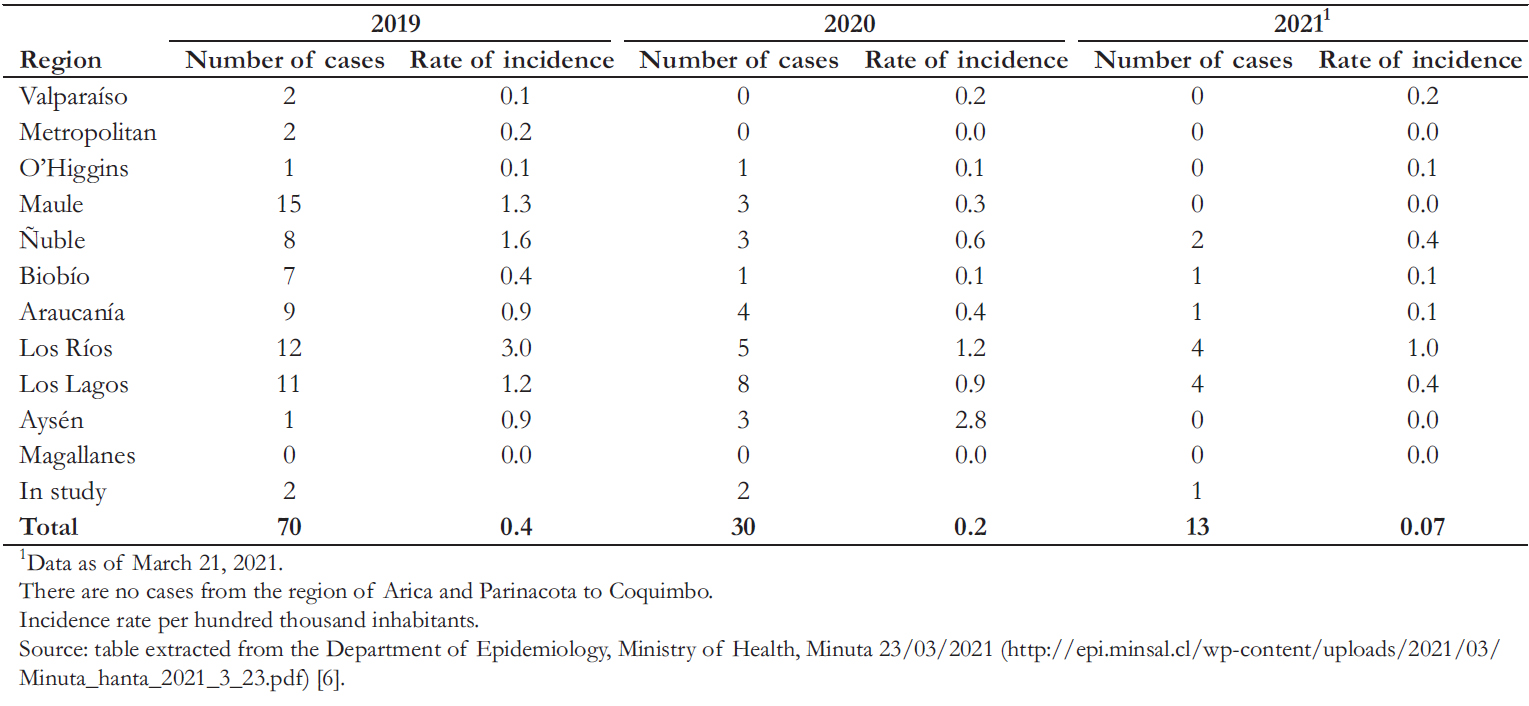
According to the Ministry of Health in Chile [6], 13 cases of hantavirus were reported between January and March 2021, compared to 30 cases in the entire 2020 year (Table 2). It should be noted that – due to the COVID- 19 pandemic and health measures proposed by the authorities – the mobility of people in these two years was reduced. In contrast, previous to the restriction measures in 2019, 70 cases were recorded.
Numerical simulations
Table 3 shows the values used in the numerical simulations. These values are extracted from other research, and some have been estimated from the available data.
We considered Nh = 100 000 as the initial human population without infected cases, and we considered Nm = 100 as the initial population of rodents, with only one infected case.
The numerical simulations expressed in graphs were elaborated via MATLAB software.
 Full size
Full size 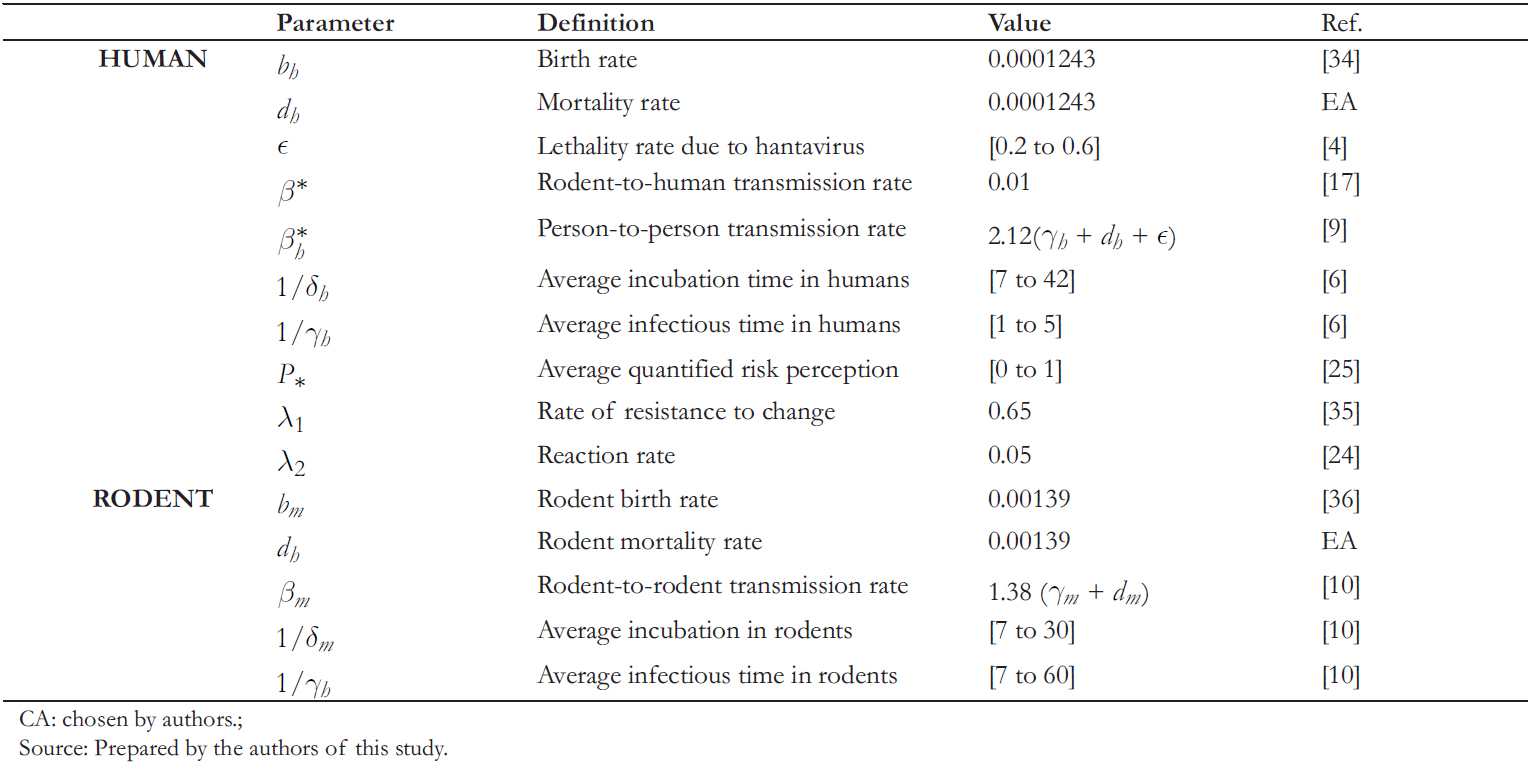 Full size
Full size Results
No person-to-person transmission
Before looking at the impact of person- to- person transmission, it is essential to visualize the rodent- to- human transmission. Through the model simulation and canceling out the rates asso-ciated with the person- to- person transmission, Figure 1 shows that in one year goes to 5.8 cases per 100 000 inhabitants (Figure 1b), with a variation during this period of +0.06 (Figure 1a). Thus, the simulations align with the real dynamics, where agriculture and forestry are the primary sources of labor.
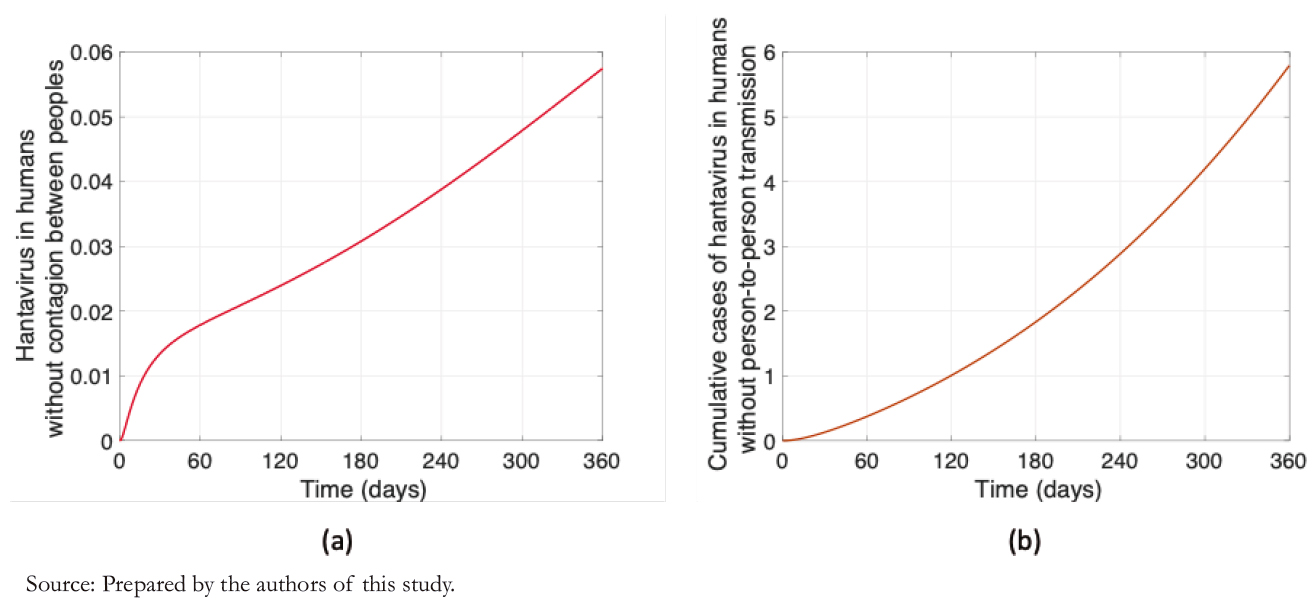
The number of infected rodents is challenging to estimate since there is no systematized registry of rodents carrying the Andes virus in Chile. However, Figure 2 shows the dynamics of the number of infected rodents that the proposed model yields. Figure 2a shows a slight variation of +2 cases, and Figure 2b shows 12 accumulated cases for one year.
Person-to-person transmission
Figure 3 shows humans' disease dynamics and risk perception, considering values shown in Table 3. Figure 3a illustrates the dynamics of hantavirus infection in humans during a year in which initially there were no infections. The maximum number of infections occurs almost at the end of one year due to the long incubation periods. It is evident from Figure 3b that risk perception has a behavior similar to the infectious curve, with daily variations.
Compared to Figures 3 and 4 shows a decimal positional decrease in the rate of resistance to change ( λ1/10 ), and an increase in the same magnitude to the reaction rate ( λ2 ∗ 10 ). It is observed from Figure 4a that the maximum contagion decreased by almost 25%, which is highly significant after increasing the risk perception considering λ1 and λ2 values. Another factor to note is that in Figure 4b (unlike Figure 3b), the daily variability in a "sawtooth" does not appear, increasing risk perception. Thus, the higher the risk perception, the lower the number of human infections. Therefore, prevention campaigns play a fundamental role in this problem.
The sawtooth presented in Figure 3b, as opposed to Figure 4b, occurs because risk perception depends mainly on two factors of our model: the rate of resistance to change and people’s reaction speed. The latter also depends on the number of human hantavirus infections. Therefore, with a higher rate of resistance to change and lower reaction speed, there is greater variability in risk perception after an increase in human infec-tions. In other words, the higher the risk perception, the "smoother" the curve.
After decreasing the rate of resistance to change and increasing reaction speed, leaving λ1 = 0, 0065 and λ2 = 1, 5 , a highly sig-nificant decrease in the maximum number of contagions is evi-dent (Figure 5a), following the increase in risk perception (Figure 5b). Unlike the other findings associated with risk per-ception (Figures 3 and 4b), Figure 5b shows that the behavior of the curve is not bell- shaped during the described period.
 Full size
Full size 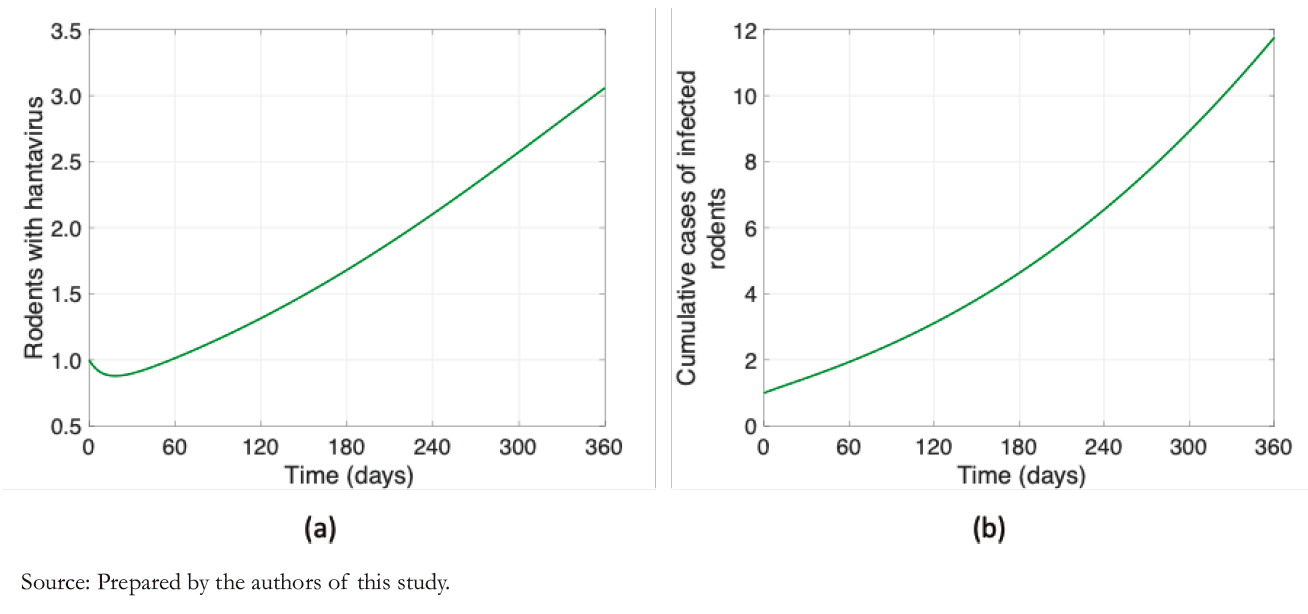 Full size
Full size 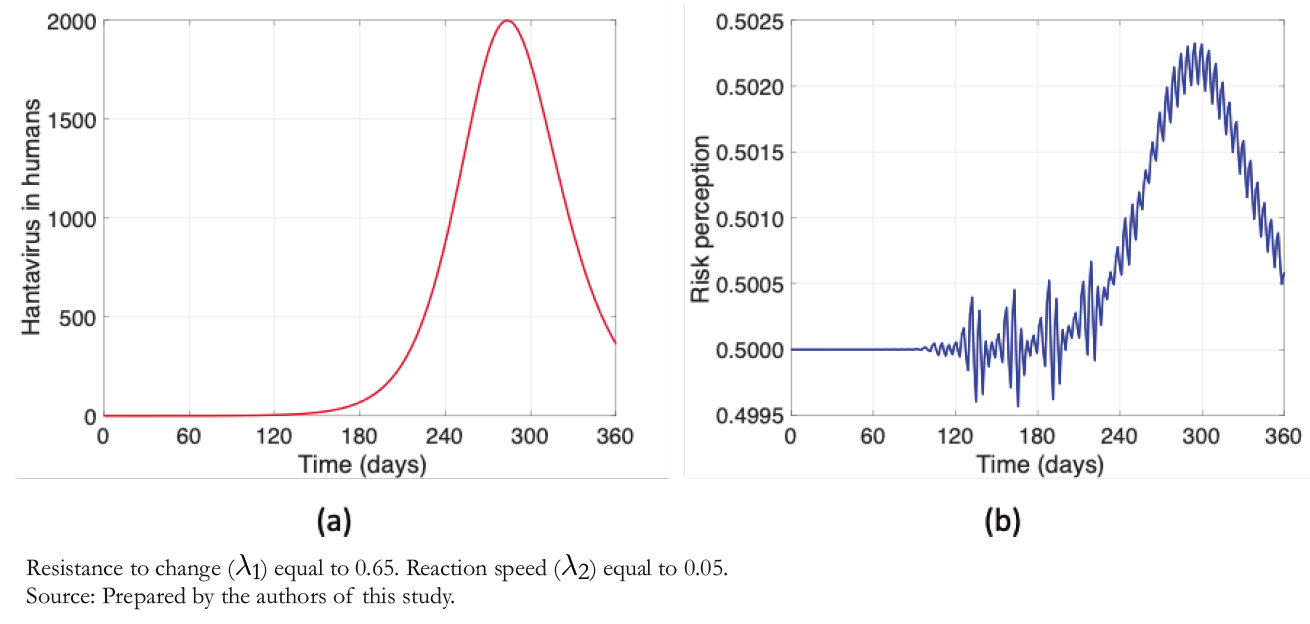 Full size
Full size 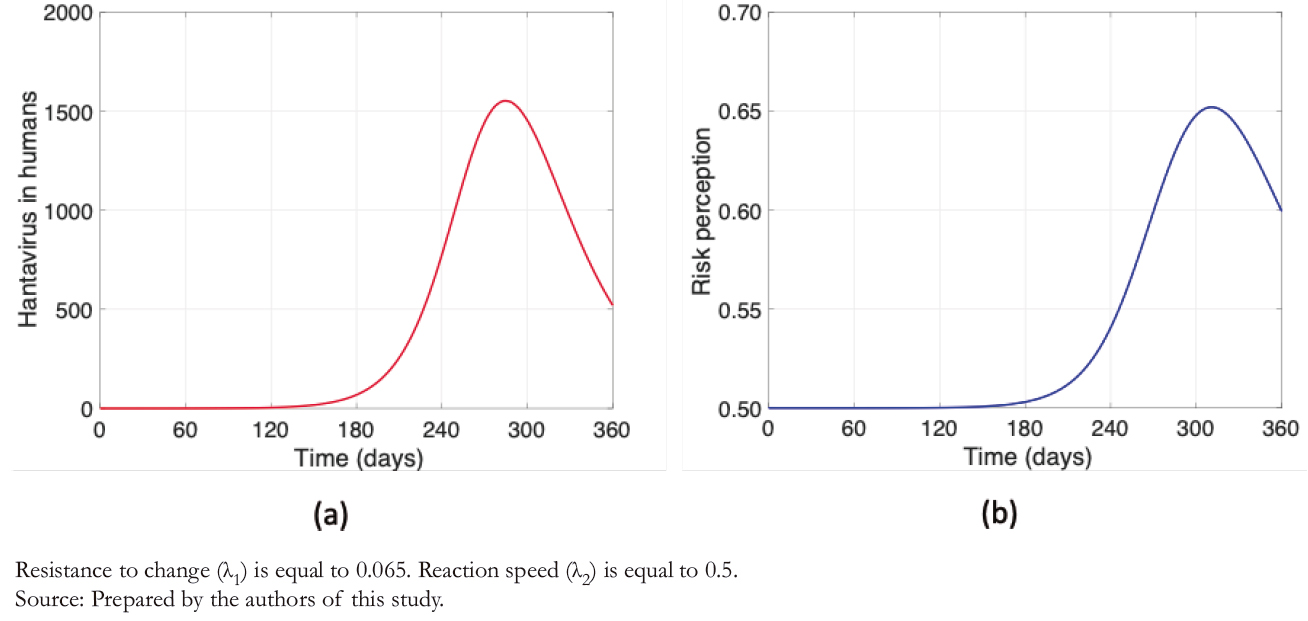 Full size
Full size 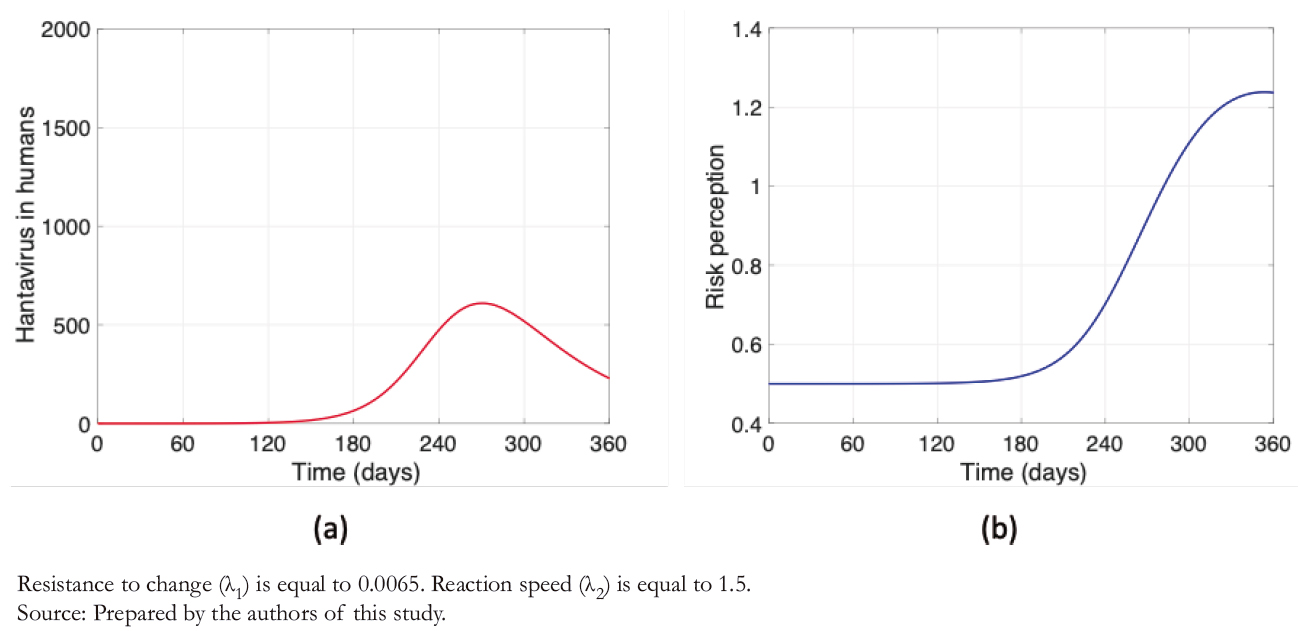 Full size
Full size Discussion
In this paper, we presented a model that expresses the dynamics of hantavirus transmission, including the possibility of person- to- person transmission and the general population’s risk perception, showing how the latter variable, associated with people’s behavior, is a determining factor in the spread of the disease. The scarce available information on person- to- person transmission and the model’s generality are the main limitations of this study. However, the model and its respective graphs associated with cases of human infection (Figures 1 and 3–5) show that surveillance and prevention strategies should be increased considering the possibility of a virus mutation. Suppose person- to- person transmission occurs and the number of infected people increases significantly. In that case, the risk of crossing infectious disease routes (such as the reported cases of COVID- 19 and hantavirus) may increase [37]. Considering the high lethality rate of hantavirus, the population would be at serious risk in areas with rodents hosting the virus [38].
Previous studies have confirmed person- to- person transmis-sion of hantavirus, particularly of the Andes virus [4],[8],[9]. A study in Argentina [9] estimated that the basic reproductive number was 2.12 before the quarantine was announced in the outbreak locality. Therefore, prevention and constant monitor-ing measures should be taken in the face of a possible virus mutation [39]. Otherwise, a new pandemic may occur, such as those already experienced by SARS- CoV- 2 and H1N1 [38].
Climate change, the accelerated expansion of agribusiness in natural reserves, the increase in temperatures, and anthropic disasters such as forest fires, produce the migration of wild rodents following the scarcity of food that occurs in their natural habitat [40],[41]. Therefore, their migration may increase the risk of rodent-to-human transmission [42].
On the other hand, a study conducted in rural Chilean commu-nities [43] evaluated the risk perception of hantavirus and showed that a high percentage of the participants knew about the disease and had heard of rodents as a natural reservoir for the virus. However, less than 50% knew that rodents and their droppings transmit the disease, and less than half of the partic-ipants mentioned fever as the main symptom. In this same study [43], it was noted that television had the highest commu-nicational impact, which may be due to low connectivity and internet access in rural areas. Participants knew that hantavirus disease could be severe and cause death. They perceived them-selves, their families, and communities as at risk of contracting this disease because of their location and occupations. Most fear the disease due to living in small communities where cases and deaths have occurred. In summary, the perception of risk towards the disease was mainly due to the impact caused by hantavirus deaths, with some – but scarce– knowledge of the disease, learned through prevention and risk communication campaigns.
At the beginning of March 2020, the first case of COVID- 19 was reported in Chile. Given the significant increase in cases, control measures were taken, mainly by reducing mobility, man-datory use of masks, and physical distancing. The reduction of people’s mobility impacted the number of hantavirus cases, as 30 cases were reported that year, representing less than half of cases reported in 2019. In 2021 restriction measures were relaxed, giving greater freedom in people’s mobility. This free-dom translated into 13 cases only for the first eleven epidemio-logical weeks, between January and March 2021 [6].
Although a series of vaccine and antiviral treatments have been developed worldwide and tested in animal models and human clinical trials for hantavirus, there are no vaccines or treatments officially approved by international organizations [44].
Ongoing prevention campaigns and effective risk communica-tion strategies are essential for controlling or reducing hantavi-rus infections in humans. These should involve community participation and be attentive to the local and cultural realities of the potentially affected populations [43],[45]. Basic research from universities and infectious disease research centers should be strengthened, focusing on implementing new strategies for viral genomic epidemiological surveillance of natural reser-voirs. Multidisciplinary work, including epidemiologists, ecolo-gists, physicians, immunologists, behavioral science researchers, should be enhanced to identify infectious sources of hantavirus [39]. The decrease in cases after increasing the perception of risk from the proposed mathematical model highlights the importance of these measures. Studies show a significant decrease in the number of cases in Chile during the periods of greater prevention campaigns through various media, posters in clinics, and educational establishments [4],[26],[27].
Conclusion
In this paper, we have presented a mathematical SEIR model to express the dynamics of hantavirus infection. We have included the possibility of person- to- person transmission and the risk perception of the population toward the disease.
The model results, via numerical simulations, were elaborated from the scarce data on person- to- person transmission and, mainly, from the information provided by the Chilean Ministry of Health, among other studies.
Through the simulations, we observed that the risk perception determines a sensitivity in the peak of the infectious curve after the variation of this latter curve. Therefore, it plays a funda-mental role in the spread of the disease.
Increasing risk perception in the general population through mitigation measures by health authorities is relevant to reducing hantavirus cases and alerting a possible new epidemic outbreak through person- to- person transmission.
Notes
Contributor roles
JPGJ: conceptualization, methodology, formal analysis, research, writing (revisions and edits), data management, data presentation, project management. MTMQ: conceptualization, methodology, formal analysis, research, writing (revisions and edits), supervision.
Acknowledgments
We thank the Vicerrectoría de Investigación y Postgrado (VRIP) and the Centro de Investigación de Estudios Avanzados del Maule (CIEAM), both belonging to the Universidad Católica del Maule.
Competing interests
The authors declare no competing interests for this article.
Funding
The authors declare no external sources of funding.
Provenance and peer review
Not commissioned. Externally peer reviewed by three reviewers, double- blind.
Language of submission
Spanish.

