Clinical reviews
← vista completaPublished on June 4, 2021 | http://doi.org/10.5867/medwave.2021.05.8202
Update on immunotherapy for renal cancer
Actualidades sobre inmunoterapia para el cáncer renal
Abstract
In the last decade, the development of immune checkpoint inhibitors have revolutionized the treatment of patients with advanced renal cell carcinoma, with the potential for dramatic changes in the therapeutic landscape. Nivolumab, a monoclonal antibody inhibitor of transmem-brane programmed cell death protein 1 (PD-1), was approved as monotherapy in 2015 for advanced renal cell carcinoma in patients previously treated with an agent targeting vascular endothelial growth factor. In April 2018, the combination of nivolumab and ipilimumab, a cytotoxic T-lymphocyte-associated antigen 4 inhibitor, was approved for patients with previously untreated intermediate- and poor-risk advanced renal cell carcinoma. Then, in 2019, combination therapies consisting of pembrolizumab (anti-PD-1) or avelumab (anti-PD-1 ligand, PD-L1) with axitinib (a vascular endothelial growth factor receptor tyrosine kinase inhibitor) were also approved for use in all risk groups. This review pre-sents a brief historical review of the association between immunology and oncology; describes essential aspects of the mechanism of action of immune checkpoint inhibitors; discusses the current evidence regarding the clinical use of different immunotherapy regimens for the treatment of patients with renal cell carcinoma, both clear cell and other histological types; and provides general information on their adverse effects. The role of appropriate patient selection is analyzed to allow individualization of therapy and improve the already promising results. Finally, per-spectives on the future use of immune checkpoint inhibitors to treat renal cancer are discussed.
|
Main messages
|
Introduction
In recent years, there has been a rapid growth in the development of antibodies that modulate specific steps of the antitumor immune response, auguring a promising future in the management of various solid tumors, including renal cell carcinoma.
Approximately 400,000 new cases of renal cell carcinoma are diagnosed worldwide each year. Almost one-third of these patients will be carriers of locally advanced or metastatic tumors [1]. Additionally, some patients operated on for localized renal cell carcinoma will develop metastases. Their evolution will vary from a few months to several years, depending on the clinical and pathologic features of the disease and their response to therapies.
The general objective of this review is to provide basic concepts on the mechanism of action of modern immunotherapy for renal cancer, as well as to provide relevant and updated information to clinicians who are not experts in the field and, as a specific objective, to constitute an aid for decision-making in the management of this pathology.
Methods
A literature search was performed in PubMed/MEDLINE for articles in English and Spanish on renal cell carcinoma-related therapies and immunotherapy, including clinical trials, meta-analyses, clinical guidelines and reviews from the last 10 years, between June 1 and August 1, 2020. Search terms were “RCC”, “renal cell carcinoma”, “kidney cancer”, “immunotherapy”, “immune checkpoint inhibitor”, “PD-1”, “PD-L1”, “anti-PD-1”, “anti-PD-L1”, “CTLA-4”, “nivolumab”, “ipilimumab”, “pembrolizumab”, “immunotherapy AND biomarkers”, “immunotherapy AND non-clear cell carcinoma”, “adverse events” and “toxicity”. In Google Scholar, a broad search was performed using the phrases “immunotherapy for renal cell carcinoma”, “immunotherapy in renal cancer”, “immunotherapy for renal cancer” and similar phrases. From the selection, primary references on the mechanism of antitumor immune response were sought. Papers presented at American Society of Clinical Oncology conferences and personal study materials (master’s degree in urologic oncology) from the last two years were reviewed in a targeted manner. Publications in languages other than Spanish and English and those not indexed were excluded.
Results
The following is a narrative synthesis subdivided into historical and general aspects of immunotherapy and topics on the treatment of renal cell carcinoma in different clinical settings.
Antitumor immune response
Immunology and oncology have been linked since the late 19th century when William Coley reported that inoculation of dead erysipelas-producing bacteria into sarcomas could reduce the tumor size [2]. This link became more apparent after 1976 when Morales et al. demonstrated the efficacy of intravesical use of bacillus Calmette-Guérin for the management of urothelial cancer [3]. Since then, a greater understanding of the mechanisms of immune surveillance and tumor development have led to important therapeutic advances.
In 2018, the Nobel Prize in Medicine was awarded to James Allison for his work on the development of antibodies that block the action of the protein cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), located on the surface of T lymphocytes, whose binding to its ligands (CD80 and CD86, or B7.1 and B7.2) translates as an inhibition of T lymphocyte activation; and Tasuku Honjo for his research focused on the programmed cell death protein 1 (PD-1) on the surface of T lymphocytes and whose blockade by antibodies favors the antitumor response (Figure 1).
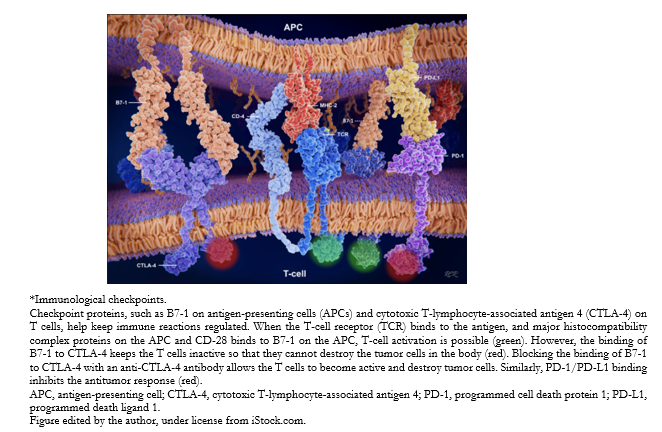 Full size
Full size The generation of immunity to cancer is a cyclical process involving immunostimulatory factors, but also other feedback factors that can halt or limit the antitumor response. This cycle can be divided into seven steps as follows:
- Begins with the release of tumor antigens.
- Which are presented by antigen-presenting cells (APCs).
- This leads to the activation of T cells.
- These move toward the tumors.
- There they infiltrates the tumors.
- They recognize the cancer cells.
- Finally end with the destruction of neoplastic cells.
The numerous factors that come into play provide a wide range of possible therapeutic targets. Thus, anti-CTLA-4 antibodies can promote step 3, and anti-PD-1/anti-PD-L1 antibodies can promote step 7 [4]. When T lymphocytes recognize surface antigens that identify a cell as cancerous, they are activated to kill it, but a braking
signal is also necessary to avoid overactivation. One such regulatory pathway occurs through increased expression of inhibitory receptors such as PD-1 by T cells. Upon binding of PD-1 to its PD-L1 ligand (normally expressed on the surface of dendritic cells and macrophages), there is a reduction in cytokine production and suppression of T-cell proliferation. It is this mechanism of adaptive immune resistance, by increasing PD-L1, that many tumors have developed to continue proliferating.
In contrast, immunotherapy aims to overcome the ability of neoplastic cells to resist this antitumor response. Thus, a molecule capable of blocking PD-1 receptor present on lymphocytes, or PD-1 ligands expressed by cancer cells, will prevent the binding of both and block the immunomodulatory signal, allowing T cells to remain active against the tumor (Figure 2).
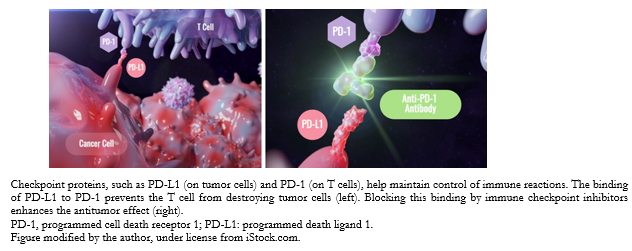 Full size
Full size Thus, anti-PD-1, anti-PD-L1, and anti-CTLA-4 antibodies have emerged as a great promise in the field of cancer immunotherapy. These immune checkpoint inhibitors have become a new modality for the management of advanced renal cell carcinoma, displacing cytokines such as interleukin-2 or interferon-α, whose results were poor [5].
Immune checkpoint inhibitors
Nivolumab, an anti-PD-1 antibody, was approved in 2015 by the US Food and Drug Administration (FDA), and in 2016, in Europe, as a second-line treatment after the use of antiangiogenics for advanced renal cell carcinoma, based on improvements in overall survival in the phase III CheckMate 025 trial. Subsequently, the combination of nivolumab and ipilimumab (immunoglobulin G1κ-like monoclonal antibody against CTLA-4) in previously untreated patients was also approved and incorporated into clinical guidelines, based on another phase III trial (CheckMate 214). Additionally, in 2019, the FDA approved the combination of pembrolizumab (anti-PD-1) and axitinib, a vascular endothelial growth factor receptor tyrosine kinase (VEFG) inhibitor, as well as avelumab (anti-PD-L1) in combination with axitinib, for the first-line treatment of patients with advanced renal cell carcinoma [6].
Immunotherapy-related adverse effects
Immune checkpoint inhibitors are associated with a particular spectrum of adverse events. These can involve virtually any organ, with toxicities including reports of endocrinopathies, diarrhea/colitis, dermatitis, hepatitis, pneumonitis, and interstitial nephritis. Headache, weight loss, hematological disorders, and joint pain, among others, are added. However, the most commonly reported immunotherapy-related adverse effect is fatigue, sometimes severe. Generally, immunotherapy-related adverse effects are transient and mild, although occasionally, they can be severe and prolonged. Management of the latter will require discontinuation of immunotherapy and the use of corticosteroids [7].
Specifically, the safety and activity of immune checkpoint inhibitors in patients with autoimmune disorders, chronic viral infections, immunosuppressive disorders, brain metastases, or pregnancy are not well established. In solid organ transplant recipients, they are contraindicated [8].
Treatment
Immunotherapeutic treatments are currently being positioned as a first-line indication in the management of advanced renal cell carcinoma, and combinations of immune checkpoint inhibitors or immune checkpoint inhibitors associated with targeted therapies are being explored to provide better results in different clinical setting [6].
Recently, combinations of antiangiogenic and immune checkpoint inhibitors have been evaluated. The biological rationale for these combinations originates from preclinical studies in models involving tumors other than clear cell and other types of cancers. However, it suggests that anti-vascular endothelial growth factor agents may enhance antitumor immunity by stimulating antigen-presenting cell function and tumor immune cell infiltration, as well as diminishing the effect of myeloid lineage suppressor cells and macrophages in the tumor microenvironment [9],[10],[11].
Targeted therapies have immunomodulatory effects within the tumor microenvironment, promoting regulatory T cells, myeloid lineage suppressor cells, and cytokines to suppress immune evasion by neoplastic cells. Therefore, a combination of both therapies could have synergistic activity. Targeted therapies include those that block tumor angiogenesis via vascular endothelial growth factor tyrosine kinase inhibitors (sunitinib, axitinib, cabozantinib, sorafenib, and pazopanib), rapamycin target inhibitors (temsirolimus and everolimus), and anti-vascular endothelial growth factor monoclonal antibodies (bevacizumab). The current evidence is mainly supported by pathophysiological rationale and retrospective studies in the absence of sufficient randomized clinical trials. For this reason, we present separately the data corresponding to different patient subgroups and clinical settings, also integrating information from the clinical guidelines of the European Association of Urology (EUA) [12], European Society for Medical Oncology (ESMO) [13], National Comprehensive Cancer Network (NCCN v2.2020) [14] and the Society for Immunotherapy of Cancer (SITC) [15]. In such a context, treatment will be defined based on each patient’s prognostic categorization.
Risk stratification
The choice of treatment should consider the prognostic risk factors on metastatic renal cell carcinoma from the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) [16]. This prognostic model integrates six adverse factors. Patients with none of these risk factors are considered low risk, those with one or two factors are considered intermediate risk and those with three or more factors are considered poor risk. These risk factors are given as follows:
- Karnofsky index (KPS) less than 80%.
- Time from diagnosis to treatment less than one year.
- Hemoglobin concentration less than the lower limit of normal.
- Serum calcium greater than the upper limit of normal.
- Neutrophil count higher than the upper limit of normal.
- Platelet count greater than the upper limit of normal.
Table 1 shows the main immune checkpoint inhibitors currently in use.
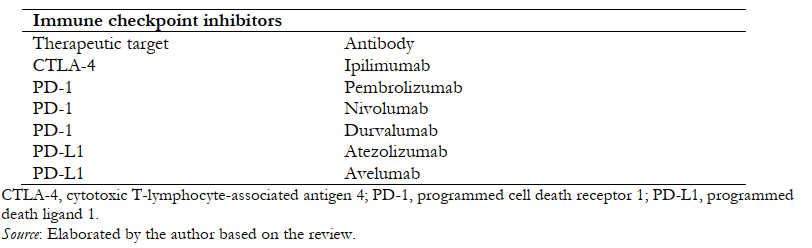 Full size
Full size Active surveillance
In asymptomatic patients with limited disease burden and no poor prognostic elements, active surveillance may represent an alternative in those who wish to defer initiation of therapy and its concomitant toxicity until progression is documented [12].
First-line immunotherapy
Compelling data on the role of immunotherapy in treatment-naïve patients came from a phase III trial that demonstrated a benefit in overall survival with the combination of nivolumab plus ipilimumab over sunitinib [17]. Subsequently, pembrolizumab plus axitinib demonstrated benefits in progression-free survival and overall survival over sunitinib [18]. Thus, for those patients with intermediate or poor risk, the preferred options include nivolumab plus ipilimumab or pembrolizumab plus axitinib. Avelumab plus axitinib is an alternative option, but so far has not demonstrated an overall survival benefit in randomized trials.
Nivolumab plus ipilimumab: In the phase III CheckMate 214 trial, 1096 patients with metastatic clear cell renal cell carcinoma, with or without prior treatment, were randomized to nivolumab plus ipilimumab versus sunitinib. Patients with brain metastases and prior exposure to targeted therapy or a checkpoint inhibitor were excluded. With a median follow-up of 25 months, results included the following: for the entire intention-to-treat study population, overall survival was increased with nivolumab plus ipilimumab (median not reached versus 32.9 months; hazard ratio: 0.68; confidence interval: 99.8%: 0.49 to 0.95). The objective response rate also increased (39% versus 32). For the 847 patients with intermediate- or poor-risk disease, there was a significant improvement in overall survival with nivolumab plus ipilimumab (median not reached versus 26 months, hazard ratio: 0.63; 95% confidence interval: 0.44 to 0.82) and in objective response rate compared with sunitinib (42 versus 27%), with a longer, although not statistically significant, progression-free survival. Adverse events occurred in 93% of patients treated with nivolumab plus ipilimumab and in 97% of those treated with sunitinib. Grade 3 or 4 events occurred in 250 (46%) and 335 patients (63%), respectively. Immunotherapy-related adverse events leading to treatment discontinuation occurred in 22% of the nivolumab plus ipilimumab group and in 12% of the sunitinib group. Eight treatment-related deaths were reported in the nivolumab plus ipilimumab group and four in the sunitinib group [17]. A report updated in 2019, with a median follow-up of 32.4 months, shows the persistence of objective response rate benefits in all risk groups [19].
Axitinib plus pembrolizumab: In the phase III KEYNOTE-426 trial, 861 patients with previously untreated advanced metastatic clear cell renal cell carcinoma were randomized to pembrolizumab plus axitinib versus sunitinib [18]. After a median follow-up of 13 months, pembrolizumab plus axitinib showed benefit regardless of PD-L1 expression and risk classification. With 27 months of follow-up, updated data from this study continue to show benefit in all risk groups and PD-L1 expression subgroups, with overall 24-month survival of 74% for pembrolizumab plus axitinib versus 66% for sunitinib. In addition, patients with an objective response rate equal to or greater than 80% show an overall survival similar to that of patients with complete response, according to RECIST v1.1 criteria [20]. On the other hand, figures from another study evaluating axitinib plus pembrolizumab in advanced renal cell carcinoma, after five years of follow-up from its phase I, show that 73% of patients are still alive, with a probability of being alive at one year of 96% and at three years of 82%; with a progression-free survival of 23.5 months (95% confidence interval: 15.4 to 30.4) and a median duration of response of 22.1 months. Thus, this combination continues to demonstrate clinical benefit without yet reaching median overall survival [21].
Other strategies: The phase III JAVELIN Renal-101 trial showed that avelumab plus axitinib significantly improves progression-free survival compared with sunitinib [22]. A second interim analysis, with a minimum follow-up of 13 months, shows a hazard ratio of 0.69 (95% inter-value confidence interval: 0.574 to 0.825; p < 0.0001) with a progression-free survival of 13.3 (95% confidence interval: 11.1 to 15.3) versus 8.0 months (6.7 to 9.8) [23]. It is a first-line option, although nivolumab plus ipilimumab or axitinib plus pembrolizumab are preferred, in the absence of mature overall survival data in which no statistically significant differences were demonstrated.
Single-agent pembrolizumab is being studied in the KEYNOTE-427 trial. This is a phase II, open-label, single-arm study in patients with advanced clear cell renal cell carcinoma (cohort A) and different histology, non-clear cell histologic renal cell carcinoma (cohort B). In cohort A, 110 patients with advanced or metastatic clear cell renal cell carcinoma were treated with pembrolizumab (200 mg every three weeks); all had measurable disease (RECIST v1.1) and had not previously received systemic therapy. Preliminary results presented in 2018, with a median follow-up of 12 months, showed an objective response rate of 38.2% (three complete responses and 39 partial responses). The response was higher in patients with intermediate-/high-risk disease than those with low-risk disease (42 versus 32%) [24]. Updated results from this cohort presented at the American Society of Clinical Oncology 2020 congress, with a median follow-up of 23.1 months (16.7 to 27.5), show that patients with a tumor burden reduction of greater than 80% have an overall long-term survival comparable to those with a complete response [25].
In the phase III IMmotion151 study, certain advantages were observed in progression-free survival (p = 0.021) with atezolizumab plus bevacizumab in the PD-L1 (+) population. However, no improvement in overall survival was demonstrated in comparison with sunitinib. So far, there is no solid evidence to support its use in advanced clear cell renal cell carcinoma, except in sarcomatoid histology, where there were better results [26].
As for combinations with greater synergistic potential, the randomized, double-blind, controlled, phase III COSMIC-313 study will evaluate the use of cabozantinib (multikinase inhibitor) in combination with nivolumab plus ipilimumab in patients with untreated intermediate- and poor-risk clear cell renal cell carcinoma (338 patients in each arm). That study started enrollment in mid-2019, and its first results are expected by the end of 2021 [27].
Alternatives in patients previously treated with antiangiogenics
The evidence supports treatment with nivolumab for patients who progress to antiangiogenic therapy without prior exposure to immune checkpoint inhibitors. This antibody improves overall survival compared with everolimus in this population. In the phase III CheckMate 025 trial, 821 patients were randomized to nivolumab or everolimus. All patients had received one or two prior antiangiogenic therapies [28]. In the updated report, with a follow-up of 64 months, patients treated with nivolumab continue to demonstrate an overall survival benefit, with 28% of patients alive at five years compared with 18% of those treated with everolimus. In addition, the percentage of patients who experienced an objective response was 23% versus 4% for everolimus, and the median duration of response for nivolumab was also maintained longer (18.2 versus 14 months) [29].
Options for immunotherapy failure
For these patients, studies suggest vascular endothelial growth factor-targeted therapy rather than a target inhibitor of rapamycin. Options include axitinib, cabozantinib, sunitinib, pazopanib or lenvatinib with everolimus [5]. In this regard, there is a report on results with tyrosine kinase inhibitors after failure of nivolumab plus ipilimumab in metastatic renal cell carcinoma as part of the CheckMate 214 trial. The median progression-free survival with first-generation (sunitinib/pazopanib) and second-generation (axitinib/cabozantinib) tyrosine kinase receptors was 8 and 7 months, respectively. These medians suggest a sustained benefit of tyrosine kinase inhibitors and support optimal sequencing investigations [30].
Interestingly, in this progression setting, the use of a second immune checkpoint inhibitor has been evaluated. Thus, a multicenter study involving 65 patients concluded that the objective response rate of immune checkpoint inhibitor-2 was 23%, comparable to that seen with immune checkpoint inhibitors after the use of targeted therapy. This was observed even with immune checkpoint inhibitors in monotherapy, and the likelihood of response was higher in patients who had initially responded to immune checkpoint inhibitor-1 [31].
Immunotherapy in renal cell carcinoma with non-clear cell histology
Data regarding the efficacy of immune checkpoint inhibitors in renal cell carcinoma other than clear cell histology and in clear cell renal cell carcinoma with sarcomatoid or rhabdoid variants are scarce. Recently, small series have demonstrated benefits in these cases [32]. In this regard, a multicenter phase II study evaluated the use of atezolizumab plus bevacizumab and showed an objective response rate of 33% and 50% in patients with renal cell carcinoma with sarcomatoid differentiation and histologic variants, respectively [33]. In the same vein, 142 patients (16%) in the IMmotion151 study had tumors with some component of sarcomatoid histology. In this regard, a subgroup analysis showed that these patients had longer overall survival and progression-free survival and a higher objective response/complete response rate when treated with atezolizumab plus bevacizumab instead of sunitinib (objective response rate of 49% versus 14% and a complete response rate of 10% versus 3%) [34].
On the other hand, cohort B of the KEYNOTE-427 trial shows promising results with pembrolizumab as first-line in renal cell carcinoma other than clear cell histology. The objective response rate in the general population was 24.8%, slightly better in papillary (25.4%) and unclassified (34.6%) and worse in chromophobe (9.5%) [35]. Updated data presented at the American Society of Clinical Oncology 2020 Congress show that a reduction in tumor burden greater than 30% correlates with better survival, becoming equivalent to that of patients with complete remission if this reduction is greater than 80%, according to RECIST v1.1 criteria [36].
Of note is the single-arm phase I/II CALYPSO study evaluating the efficacy of durvalumab plus savolitinib (MET protoncogene inhibitor) in patients with papillary variant metastatic renal cell carcinoma, regardless of whether they had received prior treatment or not. Their progress was presented at the American Society of Clinical Oncology GU 2020 Congress. In this trial, the objective response rate of durvalumab plus savolitinib was 27%, with a median progression-free survival of 5.3 months [37].
SUNNIFORECAST is a prospective randomized phase II European multicenter trial evaluating the use of nivolumab plus ipilimumab versus standard of care (sunitinib) in patients with non-clear cell renal cell carcinoma with histology other than clear cell, whose biopsies are reviewed by a referral pathologist, stratified according to all International Metastatic Renal Cell Carcinoma Database Consortium risk groups and papillary or nonpapillary histology. In phase I, nivolumab plus ipilimumab demonstrated a substantially higher objective response rate than either single agent. Its enrollment is in progress and has a primary endpoint of 12-month overall survival (n 306) [38].
General synthesis of therapeutic schemes for renal cell carcinoma
A summary of immunotherapy schemes with their main recommendations is shown in Table 2. The role of tumor expression levels of biomarkers (such as, for example, PD-L1 levels) is not well established, so they have not been included within the management decision analysis.
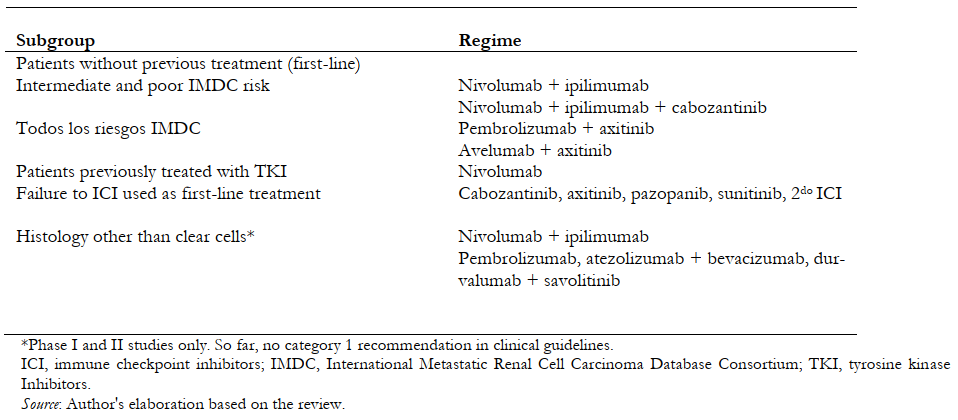 Full size
Full size Discussion
Immunotherapy has demonstrated benefits over standard therapy. However, it is still unclear which patients benefit most from combinations and what the optimal sequencing is. This is why adaptive phase III studies are being developed [39].
As more therapeutic options become available, choosing one management option over another may become increasingly complex. Because of this, it is important to establish therapeutic sequences that allow a balance between survival benefit and toxicity. In this regard, Dizman et al. propose a sequencing algorithm that, roughly speaking, proposes nivolumab plus ipilimumab as first-line, or failing that, pembrolizumab plus axitinib, for patients with advanced clear cell renal cell carcinoma or with sarcomatoid histology. After immunotherapy failure, the sequence would be cabozantinib, followed by lenvatinib/everolimus if there is progression. Nivolumab is advised in second line treatment for patients naive to immunotherapy [40].
Finally, given the benefits of immunotherapy in advanced renal cell carcinoma and in the absence of standard adjuvant therapy after surgical treatment in non-metastatic renal cell carcinoma, phase III studies comparing the use of immune checkpoint inhibitors versus observation are already underway. Such is the case for nivolumab [41] and pembrolizumab [42], both in monotherapy. Thus, the indications for immunotherapy in renal cancer are expected to expand in the coming years. In addition, likely, the development of genetic or molecular markers and the identification of predictors of oncologic outcomes will help in the optimal selection of patients and treatment schemes, consolidating immunotherapy as a new standard of management.
Strengths and limitations of this study
No recent systematic reviews on the subject were found in The Cochrane Library. The only accessible one was a review updated in May 2017, so it only includes immature data on nivolumab in advanced renal cell carcinoma and evaluated older interferon-based schemes. However, three protocols were found in PROSPERO (International Prospective Register of Systematic Reviews) as follows: (1) evaluating immune checkpoint inhibitors in previously untreated metastatic renal cell carcinoma [43], (2) regarding the efficacy and safety of immune checkpoint inhibitors combined with antiangiogenics as first-line treatment in metastatic renal cell carcinoma [44] and (3) evaluating immunotherapy versus targeted therapies for the treatment of advanced renal cell carcinoma [45]; all in early stage, with no results yet. Given this current lack of meta-analyses and systematic reviews, we believe that the present review, despite its non-systematic narrative nature with its inherent limitations and biases, provides a historical perspective and an overview of the subject that provides valuable information to base modern therapeutic decisions, especially useful for those who are entering this field of study.
Conclusions
In general terms, based on published studies, every patient with advanced renal cancer should be considered a candidate for immunotherapy with immune checkpoint inhibitors. Thus, it is important to know the basic pathophysiological aspects, the accumulated evidence, and the adverse effects of immune checkpoint inhibitors to adequately evaluate the clinical scenarios in which they are an option under a context of rational expectations.
Notes
Contributor roles
RCR is the sole author of this article.
Competing interests
The author declares that he/she has no conflicts of interest and has completed the IMCJE form. The author also declares not having received funding for the published article; not having financial relationships with organizations that could have an interest in the published article; and not having other relationships or activities that could influence the article.
Funding
The author declares that there were no external sources of financing.
Ethics
Due to the nature of the study, it did not require evaluation by an ethics committee.
Language of submission
Spanish

