Review article
← vista completaPublished on January 24, 2022 | http://doi.org/10.5867/medwave.2022.01.002525
Vitamin D supplementation in inflammatory bowel disease: a narrative review
Suplementación con vitamina D en enfermedad inflamatoria intestinal: una revisión narrativa
Abstract
Inflammatory bowel disease is a chronic disorder characterized by exacerbation and remission periods, and its worldwide incidence has increased in recent decades. Vitamin D is involved in immune regulation and improves barrier functions and intestinal microbiota. Studies have observed that high vitamin D levels decrease relapses and improve the clinical course of inflammatory bowel disease. The objective of this review was to analyze the evidence on vitamin D supplementation in adult patients with inflammatory bowel disease. Among inactive patients, the studies administrating less than 2000 international units per day of vitamin D did not find any beneficial effects. However, those supplementing 2000 international units of vitamin D per day increased serum levels and reduced disease activity. In patients with active disease, doses between 5000 to 10 000 international units per day reduced symptomatology. This review showed that vitamin D supplementation above 2000 international units per day among inactive patients with inflammatory bowel disease, and between 5000 to 10 000 international units per day among those in the active stage, shows potential benefits on the disease.
|
Main messages
|
Introduction
Inflammatory bowel disease is a chronic disorder characterized by exacerbation and remission periods of diarrhea, lower gastrointestinal bleeding, abdominal pain, fever, fatigue, and weight loss [1]. The two primary forms of inflammatory bowel disease are Crohn’s disease and ulcerative colitis, which have increased their incidence in recent decades among developed and developing countries [2].
Vitamin D is an essential fat-soluble biomolecule since it participates in many biochemical and physiological processes, including calcium and phosphorus metabolism [3],[4]. Vitamin D is ubiquitously present in tissues and is obtained through the exposure of the skin to ultraviolet light from the sun or through dietary intake of dairy products, egg yolk, red meat, and fish [4],[5],[6].
Some studies observed that vitamin D regulates the immune response [2],[7],[8] and improves the composition of the colonic microbiota and intestinal barrier, therefore playing a vital role in inflammatory bowel disease [6],[9]. Several studies have observed that high serum levels of vitamin D decrease the recurrence of inflammatory bowel disease [10],[11], and its deficiency increases the risk of clinical relapse and disease reactivation [12],[13]. Consequently, the clinical benefit seen by some authors suggests vitamin D as an attractive treatment option [9], although the optimal thresholds are unclear [14],[15]. This review aims to analyze the evidence on vitamin D supplementation in adult patients with Crohn’s disease and ulcerative colitis.
Methods
The present study corresponds to a narrative review. Primary studies published between 2015 to 2020, in English and Spanish, were included. The Web of Science and PubMed databases search engines were consulted. The following keywords were included in the search engine: "vitamin d therapy", "vitamin d supplementation", "vitamin d treatment", "inflammatory bowel disease", "Crohn’s disease", and "ulcerative colitis". Studies were eligible according to the following inclusion criteria:
- Prospective observational studies, quasi-experimental, or clinical trials.
- Studies conducted in adult humans diagnosed with Crohn’s disease or ulcerative colitis.
- Publications reported in English or Spanish.
- Studies reporting vitamin D supplementation.
Results and discussion
Inflammatory bowel disease
Inflammatory bowel disease is a chronic disorder that causes gastrointestinal tract inflammation [16],[17]. Crohn’s disease can alter the gastrointestinal wall from mouth to the perianal area, while ulcerative colitis generally only affects the colon and rectum [17],[18]. Currently, inflammatory bowel disease onset is considered to be influenced by genetic, immunological factors, environmental interactions, smoking, and drugs, among others [19],[20],[21].
Crohn’s disease and ulcerative colitis usually present with diarrhea with or without mucus and abdominal pain [22]. The severity of symptoms increases in flare-ups and may include fistulas, abscesses, and strictures in Crohn’s disease; and bleeding, perforation, pseudopolyps, and toxic megacolon in ulcerative colitis [23],[24]. In addition to the gastrointestinal damage, some patients may develop extraintestinal symptoms whose persistence is associated with exacerbations [25].
The assessment of disease activity for Crohn’s disease is performed using the Crohn’s Disease Activity Index (CDAI) and Harvey-Bradshaw index (HBI) [26]. Through the CDAI, a score lower than 50 corresponds to remission, and higher than 150 corresponds to a crisis. A crisis usually involves liquid stools, severe abdominal pain for more than seven days, and the use of antidiarrheals, extraintestinal complications, among other symptoms [26]. The HBI index evaluates daily liquid stools, abdominal pain, abdominal mass, and extraintestinal manifestations for seven days, classifying the active state with a score equal to or greater than seven [23],[24],[25],[26]. For ulcerative colitis, the Mayo Clinic severity index is used. This score also considers blood in stool and classifies crisis into mild, moderate, and severe with scores between 3 and 4, 5 and 8, and 9 and 12 points, respectively [24].
Crohn’s disease and ulcerative colitis are complex pathologies to manage during flare-ups. In this reactivation period, 20 to 40% of patients develop extraintestinal manifestations and potentially life-threatening complications [16]. Moreover, 50% of patients with Crohn’s disease and 20% to 30% of individuals with ulcerative colitis usually require surgery during activity periods [16]. In addition, patients with active inflammatory bowel disease have a 70 to 80% chance of relapse in the following year [17].
Vitamin d
Vitamin D stands out for its multiple functions in the human body, including calcium homeostasis and exerting actions as a prohormone [27]. From a molecular perspective, vitamin D presents as ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3) [4]. The latter is the primary source of vitamin D in nature since it can be endogenously synthesized with ultraviolet light from the sun [28]. Additionally, dietary intake represents 20% of the daily requirement [4],[6].
Body vitamin D is estimated by measuring the plasma concentration of 25-hydroxyvitamin D. This indicates the therapeutic window in which vitamin D can exert its phosphocalcic functions while avoiding intoxication [29]. According to most international scientific societies, blood levels lower than 20 nanograms per milliliter (less than 50 nanomol per liter) are deficient; 20 to 30 nanograms per milliliter (50 to 75 nanomol per liter) are insufficient; 30 and 50 nanograms per milliliter (75 to 125 nanomol per liter) correspond to sufficiency; 50 to 150 nanograms per milliliter (75 to 375 nanomol per liter) correlates with overdose; and greater than 150 nanograms per milliliter (greater than 375 to 500 nanomol per liter) to intoxication [29],[30].
Role of vitamin d in inflammatory bowel disease
Vitamin D has been shown to improve the intestinal barrier function, regulate the inflammatory process and balance the intestinal microbiota [31]. In the intestinal barrier, it reduces cell injury caused by inflammatory bowel disease by suppressing epithelial cell apoptosis, differential regulation of tight junction proteins (Occludin, Zo-1, Zo-2, Vinculin, and Claudin), and alteration of transepithelial resistance [13],[32]. Additionally, vitamin D promotes mucosal regeneration, decreases inflammation, and improves intestinal function via vitamin D receptor-mediated signaling pathways [10],[13]. The efficacy of vitamin D at the intestinal barrier depends on its concentration and affinity to vitamin D receptors [10],[13].
The large intestine harbors a diverse array of intestinal microbiota involved in immunity, nutrient absorption, and epithelial barrier homeostasis [10],[13]. Patients with inflammatory bowel disease have a reduced bacterial richness compared to healthy subjects [10]. Recent studies found that people with higher serum vitamin D levels have a developed and abundant microbiota, thereby reducing inflammation associated with the lower disease activity and relapses [10],[14],[34].
Regarding its anti-inflammatory role, vitamin D inhibits the synthesis of prostaglandins, decreasing intestinal permeability and hindering the proinflammatory function of immune cells [10]. Additionally, it suppresses the activity of lymphocyte subtypes, causing reduction of the inflammatory cytokines such as interleukin-2 (IL-2) and tumor necrosis factor-alpha (TNF-α) [8],[13]. On the other hand, it has been shown that this vitamin modulates the activity and differentiation of monocytes and macrophages and increases anti-inflammatory cytokines such as interleukin 10 (IL-10) and 12 (IL-12) [8],[35].
Vitamin D also contributes to reducing interleukin 17 (IL-17) synthesis, thereby increasing the anti-inflammatory activity of glucocorticoids [8]. Additionally, it favors an increase in interleukin 4 (IL-4), 5 (IL-5), 6 (IL-6), and 13 (IL-13), noted for exerting anti-inflammatory action by inhibiting TNF-α [8],[35]. Likewise, it decreases the apoptotic activity of active B lymphocytes and plasma cells by reducing immunoglobulin E secretion [8],[36].
Effect of vitamin d supplementation on inactive inflammatory bowel disease (outpatient setting)
Patients with inflammatory bowel disease are more likely to have vitamin D deficiency. These patients lack a safe sunlight exposure threshold [14] due to the increased risk of melanoma [15]. Additionally, it is common that patients fail to meet the dietary intake recommendations since some foods containing vitamin D are associated with increased symptoms [15].
Studies supplementing outpatients with doses lower than 2000 international units per day of vitamin D did not find significant changes in serum levels (Table 1). The 2016 study by Kojecky and collaborators [37] observed that supplementing 1100 international units per day did not significantly reduce inflammatory bowel disease activity through the Mayo Clinic Severity Index and CDAI, possibly because it failed to reduce serum vitamin D deficiency. On the other hand, Tan et al. [38] evaluated a dose of 1667 international units per day for 12 months, which increased serum vitamin D levels but without demonstrating significant changes in the Crohn’s disease and ulcerative colitis activity index.
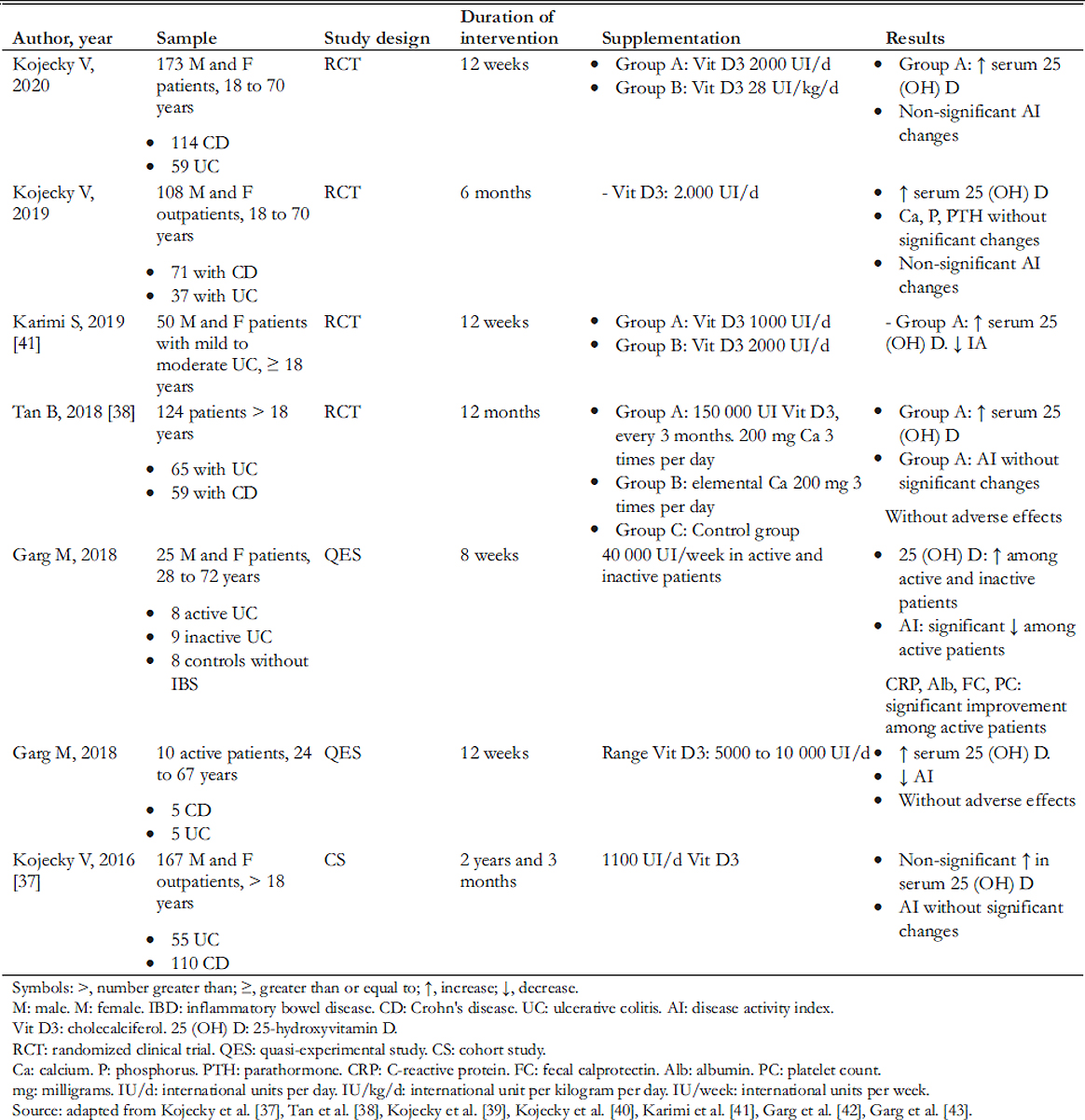 Full size
Full size Clinical trials by Kojecky et al. supplementing this vitamin D with 2000 international units per day found increases in serum levels [39],[40]. Furthermore, Karimi and colleagues [41] detected a reduction in the Clinical Colitis Activity Index (SCCAI) score in patients with ulcerative colitis, administering the same dose of vitamin D.
Effect of vitamin d supplementation on active inflammatory bowel disease
Studies that supplemented vitamin D between 5000 to 10 000 international units per day observed beneficial effects on active inflammatory bowel disease. According to Garg et al. [42],
supplementing 5000 to 10 000 international units per day of vitamin D in subjects with active inflammatory bowel disease for 12 weeks contributed to a reduction in clinical symptomatology. Garg et al. also found that the therapy did not have serious adverse events while decreasing the activity index steadily and controlling recurrence through CDAI for Crohn’s disease and HBI for ulcerative colitis [42]. Another study by Garg et al. [43] in 2018 found that 40 000 international units per week of vitamin D in subjects with active ulcerative colitis favored a reduction in intestinal inflammation by Mayo Clinic Severity Index and Colitis Activity Index. It should be noted that vitamin D levels reported by Garg et al. [43] were above the recommendations of the Spanish Society of Endocrinology [29], so extending the period of supplementation of this dose may result contradictory. Although no serious adverse effects were observed, these could develop while maintaining high serum vitamin D levels during prolonged supplementation periods.
Conclusions
A beneficial effect of vitamin D supplementation between 5000 and 10 000 international units per day has been found to reduce symptomatology and recurrences in active inflammatory bowel disease.
A similar effect has been identified with vitamin D supplementation above 2000 international units per day on the activity of inactive inflammatory bowel disease. However, supplementing 2000 international units per day of vitamin D in the inactive stage did not significantly affect the symptomatology or the activity indexes.
It is essential to constantly monitor serum vitamin D levels to avoid serious adverse effects. New studies incorporating immunological analyses and quantification of vitamin D intake may help understand the effect of supplementation in inflammatory bowel disease.
Notes
Contributor roles
MZ: conceptualization, research, data curation, preparation of the original article, review, and editing. JP: conceptualization, methodology, research, article review and editing, and supervision. CD: methodology, research, article review and editing, and supervision.
Acknowledgments
The authors would like to thank Dr. José Fernández Cao and Dr. (c). Sergio Jiménez Torres for their contribution to this article.
Competing interest
The authors completed the ICMJE conflict of interest statement and declared that they received no funding for the completion of this article; have no financial relationships with organizations that may have an interest in the published article in the last three years; and have no other relationships or activities that may influence the publication of the article. Forms can be requested by contacting the responsible author or the Editorial Board of the Journal.
Funding
The authors declare that they have no source of funding.
Ethics
This article is exempt from approval by an ethics committee because it corresponds to a secondary study.
Language of submission
Spanish.

