Current topics
← vista completaPublished on November 7, 2022 | http://doi.org/10.5867/medwave.2022.S3.2589
Decline of cytology-based cervical cancer screening for COVID-19: a single-center Peruvian experience
Declive del cribado de cáncer cervical basado en citología by COVID-19: una experiencia unicéntrica peruana
Abstract
Introduction Cervical cancer is the second most frequent malignant disease in the Peruvian female population, and the Papanicolaou test is its main screening tool. However, the COVID-19 pandemic can hinder cervical cancer screening, reducing its scope.
Objective To analyze the decline of Papanicolaou-based cervical cancer screening due to COVID-19 in a specialized hospital in Lima.
Methods We designed a retrospective study (from 2015 to 2020) on 355 029 Papanicolaou smears at the Hospital Nacional Madre Niño San Bartolomé. T-test and one-way ANOVA were used to define differences in the study period and Ljung-Box test with ARIMA (1,0,0) model to describe and forecast monthly expected Papanicolaou smears for 2020.
Results Throughout the six years of the study, the average Papanicolaou smears was 59 171.5 ± 8898.7 per year. However, in 2020 only 16 273 (4.58%) Papanicolaou tests were performed with a monthly mean of 1356.1 ± 684.2 (95% confidence interval 149.7 to 2861.9) (p < 0.001). The forecast showed 66 960 Papanicolaou smears for 2020 and a monthly mean of 5580 ± 129.3. Actual screenings during that year were only 16 273 Papanicolaou smears, resulting in a 76.7% reduction in cervical cancer screening during the pandemic.
Conclusions Our results suggest a dramatic decrease in cervical cancer screening based on Papanicolaou smears during 2020 in Peru due to prevention and control measures against COVID-19.
Main messages
- Cervical cancer is a leading cause of female morbidity and mortality in Latin America.
- The COVID-19 pandemic has limited cervical cancer screening due to restrictions imposed by the state of emergency.
- The COVID-19 pandemic has significantly reduced Papanicolaou smear screening from 59 171.5 tests to 16 273 tests per year.
- There has been a 76.7% reduction in cervical cancer screening in 2021 in a Peruvian specialized hospital.
- Screening programs must improve their coverage and surveillance of cervical disorders and cancer in Latin America.
Introduction
Cervical cancer is a leading cause of female morbidity and mortality worldwide and a major public health concern in the Americas region [1]. Although cervical cancer screening systems are progressively shifting towards molecular testing for the identification of human Papillomavirus (i,e., the leading infectious agent linked to the development of squamous intraepithelial lesions into cervical cancer), most countries continue to focus their diagnostic strategies on the Papanicolaou smear test [2]. The Papanicolaou test has led to a dramatic reduction in cervical cancer worldwide, and it is highly specific, simple, and cost-effective, but it has a low sensitivity and depends on human error [3,4].
More than ever, public health programs urgently demand timely cervical cancer screening in primary health care due to the new coronavirus (COVID-19) pandemic, which has hindered health care tools to prevent cervical cancer [5]. In addition to all the pre-existing barriers to cervical cancer screening in low- and middle-income countries, COVID-19 has led to the redistribution of resources in the face of pandemic demand for prioritized services. Among other factors, it is essential to consider the reduced access to health care facilities due to the restriction of means of transport (land, air, and sea, regional and international), the collapse of insurance coverage and health services, the impoverishment and greater vulnerability of the patient, insufficient communication and technology strategies, and the decrease in human resources (specialized professionals over 60 years of age and with comorbidity factors) [6,7].
Since the beginning of the pandemic, the number of patients who have stopped attending health centers for early detection of cervical cancer by Papanicolaou smears is not yet available for all low- and middle-income countries. Consequently, it is not possible to determine an approximate number of potential cases of squamous intraepithelial lesions and cervical cancer that went undiagnosed due to hospital closures in the face of the pandemic and the crisis in cervical cancer screening programs [8]. . Therefore, this study aims to analyze the decline of cervical cancer screening based on the Papanicolaou test due to COVID-19 in a specialized hospital in Lima, Peru.
Methods
Study design and study period
We designed a retrospective longitudinal observational study at the Hospital Nacional Madre Niño San Bartolomé, following the recommendations for observational studies of The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement [9]. This study analyzed monthly, and annual Papanicolaou smears between 2015 and 2020. The Hospital Ethics Committee approved this work.
Eligibility criteria
We included 355 029 Papanicolaou smears referred to the hospital’s Anatomic Pathology department from health networks, covering about 35% of the female population of Lima [10]. The hospital has a counter-referral system, which guarantees continuity of care for patients according to their needs by transferring them from the community or health facility with the lowest to the highest resolution capacity.
Data analysis
For all analyses, we used the statistical software IBM SPSS v25.0 (Armonk, US). Initially, we performed a descriptive analysis using central tendency (mean and median) and dispersion (standard deviation) statistics for each year. To determine differences between study periods, we used the Student’s T-test and analysis of variance (ANOVA one way), considering a p-value threshold of 0.05 as statistically significant. Also, stationarity was verified by the autocorrelation function and the partial autocorrelation function using the Ljung-Box test [11]. The statistical software fitted the model for the time series data, being the autoregressive integrated moving average (ARIMA) 1,0,0 the most suitable to describe and estimate the series of the monthly performance of Papanicolaou tests as a function of time unit, represented by linear graphs.
Results
The average number of Papanicolaou tests was 49 774 ± 30 439.2 per year (range: 6265 to 75 161). The monthly and annual behavior of Papanicolaou tests performed during the six years of the study showed that in 2015, 75 161 (21.17%) tests were performed with a monthly mean of 6263.4 ± 153.7 (95% confidence interval: 5925 to 6601.8). Similarly, 74 265 (20.92%) Papanicolaou tests with a monthly mean of 6188.8 ± 524.6 (5034.1 to 7343.3) were performed in 2016, 62 650 (17.65%) with a monthly mean of 5220.8 ± 266.9 (4633.4 to 5808.3) in 2017, 64 921 (18.29%) with a monthly mean of 5410.1 ± 298.9 (4752.2 to 6067.9) in 2018, and 61 759 (17.40%) with a monthly mean of 5146 ± 185.3 (4738.5 to 5554.6) in 2019.
At the beginning of the lockdown in March 2020, 15 814 Papanicolaou smears were performed. In 2020, 16 273 (4.58%) were completed with a monthly mean of 1356.1 ± 684.2 (95% confidence interval 149.7 to 2861.9), showing a decrease of more than 42 000 tests compared to the annual mean of previous years. We found no difference in the number of tests between pre-pandemic years (p > 0.05) but a difference in the number of Papanicolaou tests during 2020 compared to previous years (p < 0.001) (Figure 1).
Distribution of Papanicolaou smears performed between 2015 and 2020 at the Hospital Nacional Madre Niño San Bartolomé.
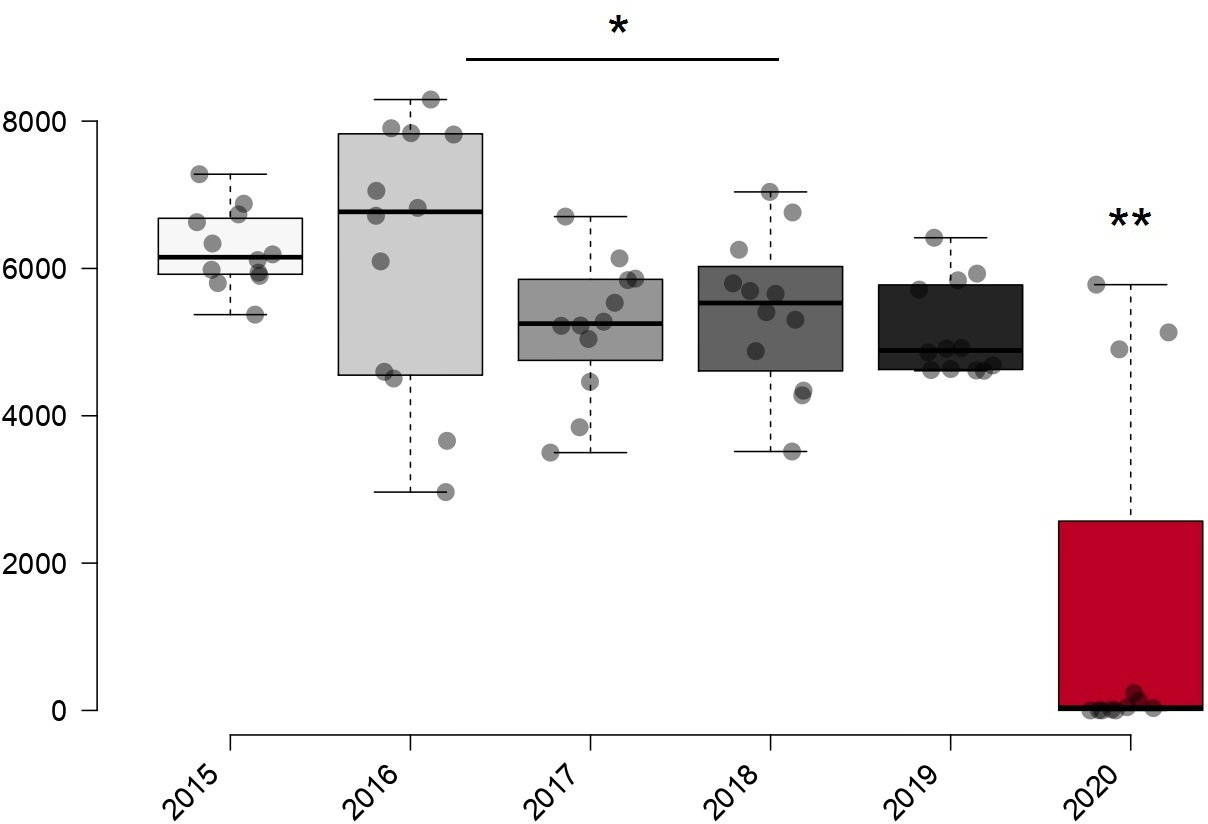
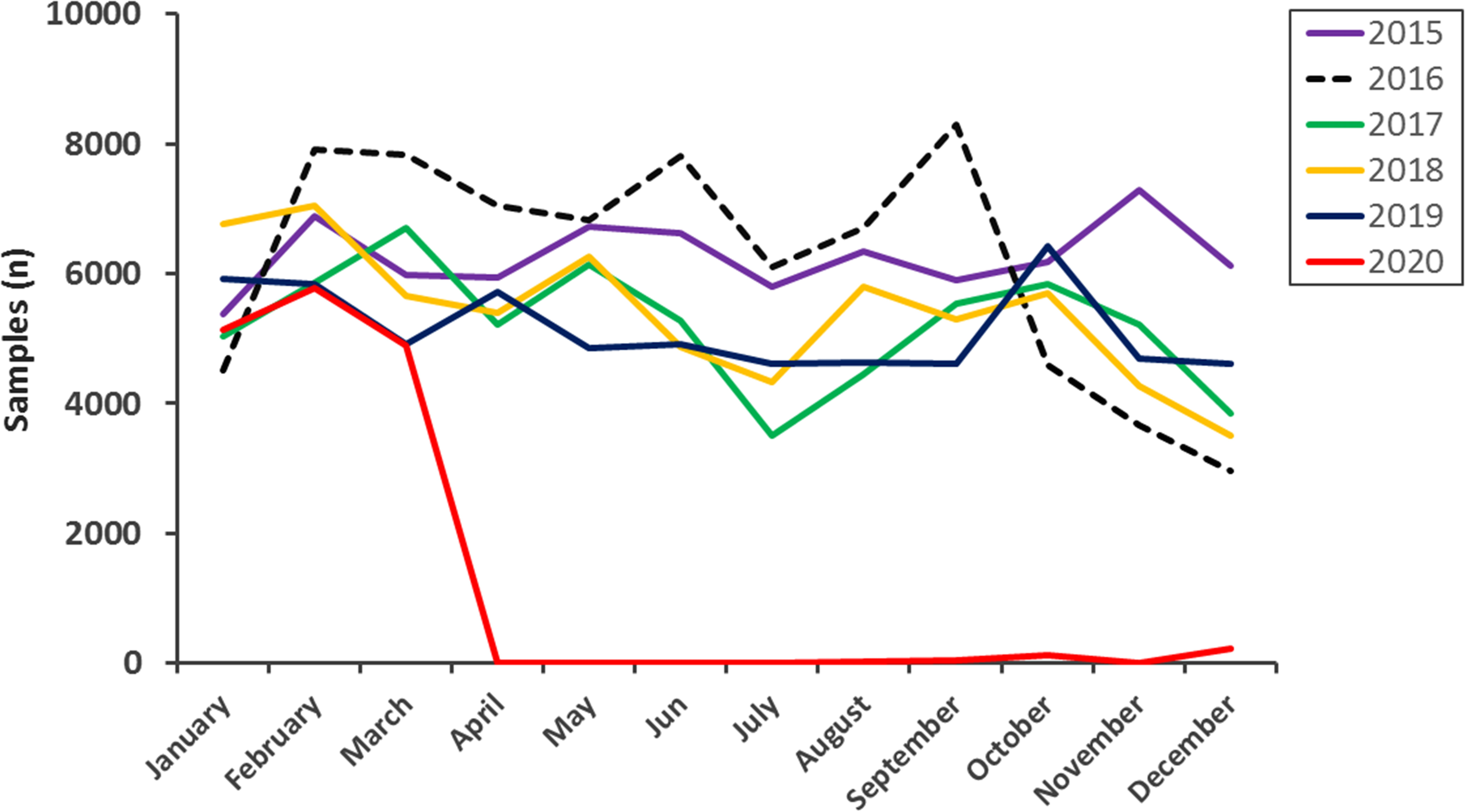
It is undoubtable that during 2020 there was a decline in the number of cervicovaginal smears performed, as the decree of COVID-19 prevention measures reduced the flow of care for other diseases such as cervical cancer. Thus, the Peruvian government opted to declare a state of health emergency and mandatory isolation on March 15, 2020, prioritizing patients infected with SARS-CoV-2 but neglecting the diagnosis of other diseases. As a result, a significant decrease in cervicovaginal smears can be observed from March 2020 onwards (Figure 2).
Monthly distribution of Papanicolaou tests at the Hospital Nacional Madre Niño San Bartolomé from 2015 to 2020. A decrease in testing with contingency measures against COVID-19 is observed (red line).
 Full size
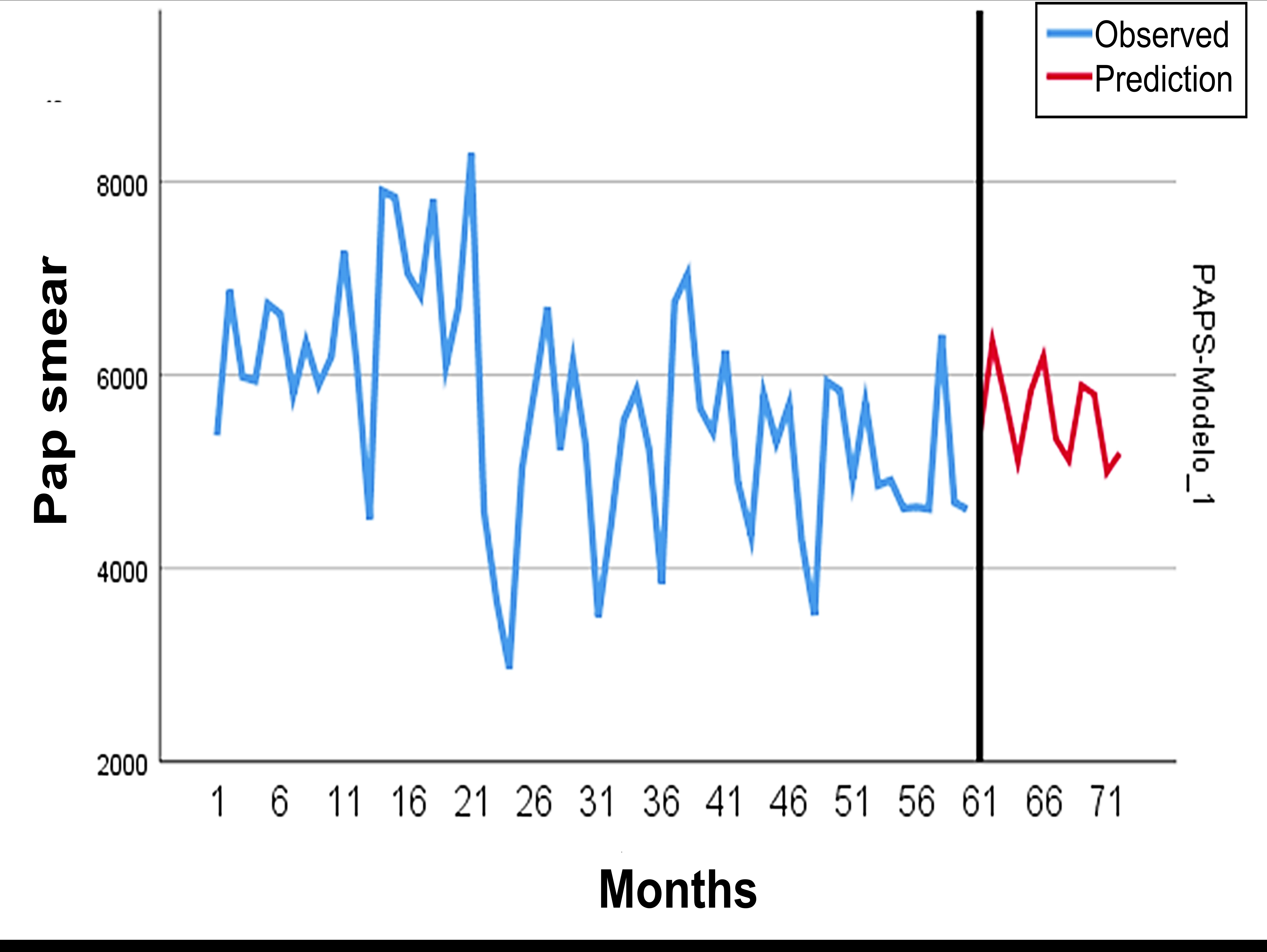
Full size Time series analysis showed periodic seasonal fluctuations and data tendency (Figure 3Figure 3). Through the ARIMA (1,0,0) model, we estimated the monthly incidence of Papanicolaou smears during 2020. Our findings show a stable tendency over time, with a total of 66 960 Papanicolaou smears for 2020 and a monthly mean of 5580 ± 129.3 (95% confidence interval: 5295.42 to 5864.58). However, when compared to the actual Papanicolaou tests performed this year (16 273), we found a difference of 50 687 tests unperformed (76.7% reduction in cervical cancer screening, p < 0.001).
Sequence of predicted Pap smears for the 2020 period according to the ARIMA (1,0,0) model. Note the trend in Papaniclolaou smears (cervicovaginal smears) prior to 2020 (blue line) and the forecast for 2020 (red line) without the effect of prevention measures by COVID-19.
Pap, Pananicolaou.
Discussion
In this study, we found a significant decline of 76% in cervical cancer screening based on Papanicolaou smears due to the confinement measures taken in Peru for COVID-19 during 2020.
The impact of the COVID-19 pandemic has been overwhelming for our health system: the decline and postponement of cervicovaginal screening for cervical cancer prevention have turned early and timely detection into late and untimely screening, contributing directly and indirectly to the morbidity and mortality of this disease. Our findings align with Martelucci et al. [12], who found that the number of smear tests per hour has significantly reduced from 4.1 to 3.6 per hour due to the COVID-19 pandemic. Likewise, Nogami et al. [13] reported a reduction of about 50% of Papanicolaou smears during the pandemic compared to 2019.
Many diseases have been neglected since the emergence of SARS-CoV-2 in December 2019. The impact of this setback on cervical cancer has been abysmal, as shown by our results. Since the beginning of the COVID-19 lockdown, screening rates for Papanicolaou smears have declined dramatically without being able to balance out by the end of 2020 (Figure 2). This decrease in cervical cancer screening is consistent with the results of Ivanuš et al., [14] who report a 92% decline and a reduction in treatment by 15%, mainly in women aged 30 to 39 years, due to the impact of the measures taken by COVID-19.
Hopes of returning to a regular cervical cancer screening are still uncertain for low- and middle-income countries. In Peru, a cytotechnologist could read 48 cervical smears per 6-hour working day [15]. However, this primary healthcare tool has been displaced by the justified fear and anguish of a population still terrified to approach a health center to avoid infecting themselves or their family members with SARS-CoV-2. This crisis of reduced cervical cancer screening is already generating inter-population disparities. In this vein, it has recently been reported [16] that COVID-19 has reduced cervical cancer screening by 82%, 84%, and 92% in black, Hispanic, and Asian Pacific Islander women, respectively.
Given this situation, it is urgent to reintroduce mass screening based on Papanicolaou smears and reduce pre-existing inequalities [17]. In this line of action, a post-COVID-19 cervical cancer risk stratification scenario must be organized. Australia’s successful cervical cancer prevention program has faced challenges during the pandemic. Its National Cervical Screening Program targeting women aged 25 to 74 years with human Papillomavirus testing, has been drastically affected during the lockdown in the first and second waves of the COVID-19 pandemic [18], unlike the colon cancer screening program, which uses self-testing as a tool to improve their screening activities [19].
Cervical self-testing proved to be as precise as hospital-based sampling by health professionals [20]. As an accepted and growing resource in various parts of the world, self-testing can extend its benefits from human Papillomavirus screening to liquid-based or conventional Papanicolaou smears, thus increasing the possibilities of continuing the screening process during lockdowns (avoiding the risk of SARS-CoV-2 spreading) and ensuring the continuity of cancer prevention.
In Malaysia, due to the imposition of the national lockdown called "Movement Control Order" on March 18, 2020, the feasibility of conducting human Papillomavirus DNA screening by self-testing during the COVID-19 pandemic was determined as a primary test. The results of this program in 55 women showed a 1.85% prevalence of oncogenic human Papillomavirus, and 40% of patients preferred cervicovaginal self-testing [21]. In that sense, this discussion opens the opportunity to improve cervical cancer prevention programs with cervicovaginal self-testing. However, further studies are needed to understand its performance, efficiency, and limitations in times of mobility disruption, such as during the COVID-19 pandemic [22].
Finally, a possible three-phase approach for early detection of human Papillomavirus under the COVID-19 circumstances has been established. The first phase refers to primary screening in the general female population. Self-testing appears to be a fundamental tool as it confers greater security and peace of mind, may be equivalent to a medical examination, and has proven practical and easy to teach. The second phase corresponds to the triage of women with tests positive to determine the necessity of treatment. And lastly, phase three corresponds to the treatment of women with positive triage at increased risk of precancerous lesions and cervical cancer [23].
Conclusions
Our results suggest a dramatic decline in Papanicolaou-based cervical cancer screening during 2020 in Peru due to prevention and control measures against COVID-19. Compared to previous years, the average annual Papanicolaou testing has been reduced by more than three quarters, with no improvement in testing volume by the end of 2020.
Cervicovaginal self-testing is discussed as a new diagnostic methodology that shifts the paradigm from the doctor-patient system established primarily by the Papanicolaou test to a stand-alone system using molecular detection of human Papillomavirus in conjunction with the Papanicolaou test. However, it is essential to assess the impact and perceptions of women on healthcare policies and interventions for future uptake in the health system.

