Comunicaciones breves
← vista completaPublicado el 20 de diciembre de 2023 | http://doi.org/10.5867/medwave.2023.11.2787
Respuesta inmune humoral por exposición a antígenos de SARS-CoV-2 en adultos con riesgo cardiovascular: The COmmunity Cohort
SARS-CoV-2 humoral immune response in patients with cardiovascular risk factors: The COmmunity Cohort Study
Abstract
Based on the recommendations of the Vaccine Advisory Committee and the National Immunization Program, Chile implemented an early vaccination process of the population with vaccines from different laboratories. The study of neutralizing antibody levels in different population subgroups contributes to the establishment of correlates of protection against SARS-CoV-2 infection. In 2022 and 2023 we set up a community cohort of 914 adults with cardiovascular risk factors. In this cohort we are measuring the humoral immune response to exposure to SARS-CoV-2 antigens, either by vaccines or infection, as well as the incidence of COVID-19 and other adverse events. This cohort, which we call The COmmunity Cohort, is providing us with valuable information on the levels of neutralizing antibodies in these individuals and their degree of protection against COVID-19.
Main messages
- The study of neutralizing antibody levels in different population subgroups is important because it contributes to establishing correlates of protection against SARS-CoV-2 infection.
- Studies that evaluate the strength and duration of the immune response in relation to booster doses or their scheduling are scarce.
- This cohort will contribute to the scientific knowledge of SARS-CoV-2 vaccines and the humoral immune response of a rarely studied Chilean population with prevalent comorbidities.
Introduction
In response to the Vaccine Advisory Committee’s recommendations and the National Immunization Program, Chile implemented an early vaccination campaign for the population with vaccines from different laboratories, different presentation platforms, and different SARS-CoV-2 antigens. By December 2022, 94.28% of the Chilean population had received the complete primary vaccination schedule. On the other hand, Chile also reports excess mortality during pandemic years that can be attributed to COVID-19 directly, or indirectly due to less control and treatment of acute and chronic diseases during that period [1]. This excess mortality is still present in 2023, despite high vaccination rates and very significant decreases in disease lethality.
The evolution of the COVID-19 pandemic in Chile and in the world raises several important questions, among which the following can be mentioned:
-
In view of the vaccination campaigns carried out in the country and with the current immunization levels in the population, would the inclusion of booster doses of the anti-SARS-CoV-2 vaccine in the National Immunization Plan be justified or not?
-
With what administration schedule?
-
What should be the prioritized population subgroups, or, rather, should there be a population-wide immunization approach?
-
Should we integrate the SARS-CoV-2 vaccine with influenza vaccines?
-
Are the antigens with which people in Chile have been vaccinated the most adequate to generate protection against future variants?
-
What will be the impact of the bivalent vaccine, and will it be associated with a clinically significant gain over immunity already achieved with previous vaccines, infections, or both?
These knowledge gaps are directly related to the effectiveness (protection of the population) and social acceptability (or its opposite, vaccine hesitancy) of preventive interventions deployed at the primary healthcare level.
Throughout the SARS-CoV-2 pandemic, vaccines have been developed using different platforms and different virus antigenic components, or the whole virus. The challenge now is to develop a pan-sarbecovirus vaccine that can prevent morbidity and mortality caused by any known or future variants of this virus, as well as infection by other sarbecoviruses that have not yet emerged [2]. The efficacy of such a vaccine (protecting against any coronavirus) cannot be established in phase 3 clinical trials against placebo but will have to use one or more correlates of protection that take into account the complexity of the immune response [3]. A correlate of protection is an immunological function causally or correlatively associated with vaccine-induced clinical efficacy [4].
The study of neutralizing antibody levels in different population subgroups is important because it contributes to establishing the correlates of protection against SARS-CoV-2 infection (mild, moderate, or severe). Both in Chile and worldwide, the presence and strength of neutralizing antibodies after exposure to vaccines or natural SARS-CoV-2 infection have been studied and quantified [5] only in descriptive investigations, with no long-term follow-up and often only with small sample sizes. However, unlike what happens with influenza [6], the search continues for a marker that is easily identified in the serum of individuals and correlates with protection. This marker is usually an antibody that expresses a correlate of protection against infection, symptomatic disease, severe illness, or death [7].
The COmmunity Cohort Study
Studies evaluating the strength and duration of the immune response following booster doses or their timing are scarce. There is also scarce evidence on the immune response (humoral or cellular) in specific subpopulations characterized by greater vulnerability, such as adults with cardiovascular risk factors in an outpatient or community setting. Accordingly, during 2022 and early 2023, we established a cohort of 914 adults enrolled in the Cardiovascular Health Program of three community healthcare centers in Chile to evaluate this high risk group. The methods of this study, including sample size calculation, enrollment and follow-up strategies, were published in the study protocol [8]. In this cohort we are measuring the humoral immune response to SARS-CoV-2 antigen exposure, either by vaccines or infection, as well as the incidence of COVID-19 and other adverse events. This cohort, which we call The COmmunity Cohort, is giving us valuable information on neutralizing antibody response in these more susceptible individuals and their degree of protection against COVID-19.
We chose this population because, since the beginning of the pandemic, numerous publications have concluded that a higher cardiovascular risk is associated with higher morbidity and mortality due to COVID-19 [9,10]. In Chile, there is a Cardiovascular Health Program that treats adults with atherosclerotic cardiovascular diseases, hypertension, type-2 diabetes mellitus, dyslipidemia, and smoking. It was, therefore, of interest to focus on this population. So far, all we know about the humoral immune response to SARS-CoV-2 is in populations not characterized by risk but by data accessibility or convenience.
Figure 1 shows the eligible population, those enrolled per primary healthcare center, and the main results of enrollment and follow-up. Preliminary baseline analysis of 682 participants showed that female sex was predominant (76%), 46% were between 50 and 70 years of age, 81% were overweight or obese, 55% had only one cardiovascular risk factor, 31% had two associated risk factors, and 14% had three or four risk factors. The most frequent risk factor was hypertension (75%), followed by diabetes mellitus (40%) and dyslipidemia (36%). Smoking is the least frequent cardiovascular risk factor, being found in only 5% of the participants. The vaccination coverage profile of the cohort mirrors the well-known national coverage. Only 2% had not been vaccinated; although small, this group can contribute valuable data. Regarding history of COVID-19 prior to cohort entry, 64% had no history of COVID-19, 29% had mild (as defined by the World Health Organization) or no symptoms, and 7% had moderate or severe COVID-19. On the other hand, to date, 10% of the cohort has had mild COVID-19 during follow-up. Losses or withdrawals have been low, only 3%.
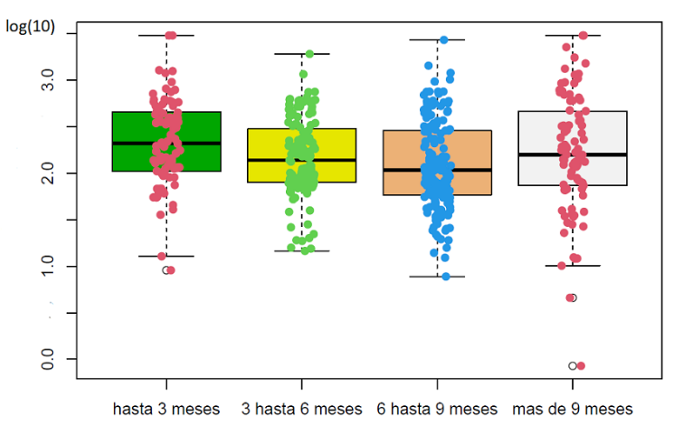
Neutralizing antibody levels are being measured with a quantitative chemiluminescence detection assay. The analysis considered the time elapsed between the last vaccine received and the date of sample collection. Figure 2 shows that the drop in levels has been rather discrete, considering that finding changes on a logarithmic scale is usual.
Constitution and follow-up of the cohort.
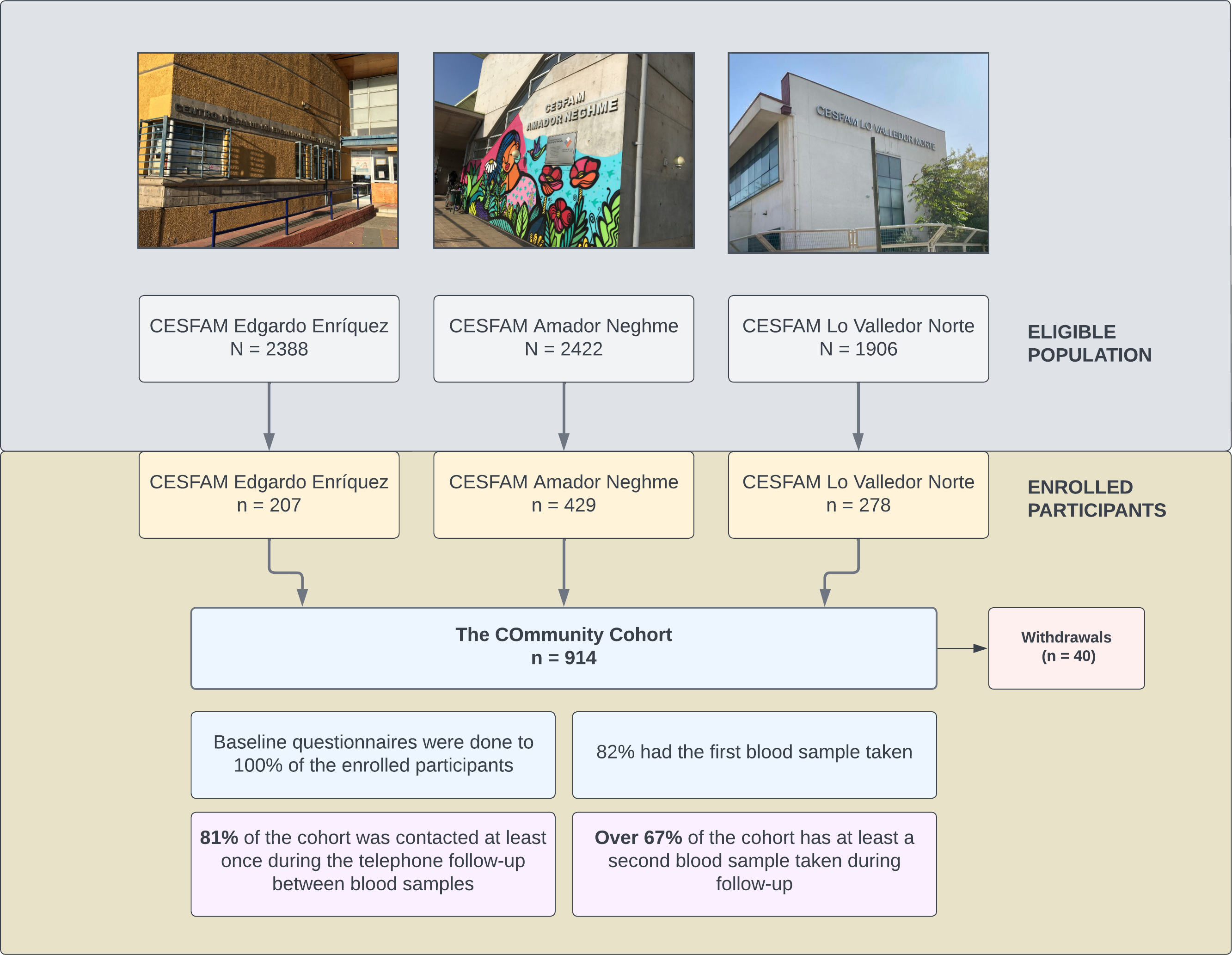
Source: Prepared by the authors based on the results of the study.
Neutralizing antibody levels expressed in index units/ml, measured by the sCOVG assay (n = 469).
Conclusions
Longitudinal studies are an essential tool for COVID-19 epidemiological surveillance and pandemic response preparations. Prospective cohort studies are essential for probing questions of harm (or protection, their complement). They are also the best design for establishing associations between risk factors and exposures of interest, such as different types of SARS-CoV-2 vaccines.
The cohort of 914 adults with cardiovascular risk factors is currently constituted and under follow-up. The study is called the COmmunity Cohort Study, and more information can be accessed at
The results obtained annually are relevant input for decision-making on using COVID-19 vaccines in Chile, a key healthcare policy. Likewise, other lines of research can be opened from the stored serum and blood samples of the cohort.

