Short communications
← vista completaPublished on March 29, 2019 | http://doi.org/10.5867/medwave.2019.02.7603
Handsearching and electronic search of clinical trials in Cuban medical journals: analysis of terminology
Búsqueda manual y electrónica de ensayos clínicos en revistas médicas cubanas: análisis de la terminología
Abstract
Introduction Clinical trials are the gold standard for testing the efficacy and safety of interventions. On their own they may not be enough to reach definitive conclusions, but they are the basis for systematic reviews that synthesize the results of several studies. However, once clinical trials have been published, a poor description of the study design and lack of specific key words and descriptors make it difficult to retrieve them by electronic searches, thus requiring hand searching.
Objectives To compare the retrieving capacity between hand search and the multiple strategies of electronic searches for identifying clinical trials in Cuban medical journals, and to determine the terminology used for describing these studies.
Methods We combined electronic searches in the Scientific Electronic Library Online of Cuba (SciELO Cuba) and Cuban database Cumed with hand search using the Cochrane guide to locate trials in three Cuban journals in the period 2000-2012. We identified the significant terms included in the title, summary, keywords and methods of each article according to Cochrane, CONSORT, and the health sciences thesaurus.
Results We identified 50 trials by hand search; four of them were retrieved by electronic search through SciELO Cuba (8%) while none was found through Cumed. The less descriptive sections were the title and the keywords. More keywords than authorized descriptors were used; the only specific concepts used in over half of the retrieved trials were “controlled” (60%), and “study groups” (52%); “randomi-zed” was used in 50% of the retrieved documents. While more specific, the terms “clinical trial”, “phase”, and “clinical trial registration” were not used.
Conclusions Compared to hand searching, electronic searches are insufficient to identify clinical trials. Therefore, the combination of the two meth-ods is necessary to reach higher retrieval rates. The terminology used to describe clinical trials in the selected journals was deficient due to underutilization of the health sciences thesaurus.
Introduction
The clinical trial is an experiment that prospectively assigns humans to intervention, concurrent comparison or control groups to study the cause-effect relationship between a medical intervention and a health outcome. In this context, medications, surgical procedures, devices, behavioral treatments, and changes in the care process, among others, are considered as interventions[1]. One of the most used classifications of these studies, according to the purpose they pursue, is by phases from I to IV, in addition to other combinations between them.
Clinical trials are considered the gold standard for assessing the efficacy and safety of interventions[2] and are an essential contribution to the consolidation of evidence, so disclosing their results constitutes a scientific and ethical obligation required by the Declaration of Helsinki in its principle number 27[3]. They are valuable as independent primary studies and are essential for the development of synthesis resources.
Its publication, like any other research, should meet quality requirements such as a complete description of the study design. In this description, the author participates, first, by proposing a representative title, descriptive keywords of the content, the abstract and the methods section with sufficient information. This process indirectly also involves referees, editors and journal director, as well as information professionals when they are included in the indexing and summary services. For this purpose, some tools facilitate the description of the studies as a guarantee for their subsequent search and recovery.
Among these tools, highlights controlled vocabularies containing subject headings, definitions, and synonyms, such as medical thesaurus: Descriptors in Health Sciences (DeCS)[4] and Medical Subject Headings (MeSH)[5]. These documents are dictionaries that translate natural language keywords into a unique vocabulary used by information systems and establish authorized medical descriptors for the indexing of literature in this branch of science.
The Consolidated Standards of Reporting Trials, known internationally as CONSORT, which establishes the descriptive elements of a clinical trial report, is another important instrument to normalize and consolidate quality, guarantee a sufficient description and facilitate the understanding of the study. Among other aspects, CONSORT suggests identifying the experimental methodology in the title, specifying "randomized", or specifying the type of design (parallel, factorial) in the methods section[6].
Both the CONSORT in its 2010 version[6] and the Declaration of Helsinki itself since 2008[3] include the registration code as another element that identifies a clinical trial. The registration of an essay, an initiative proposed by the International Committee of Medical Journal Editors and supported by the World Health Organization, promotes the registration of the study in "a public database, with information on the design and objectives, before to recruit the first patient as a prerequisite for the publication "[7].
The correct description of a study from its conception facilitates its processing in bibliographic databases and its subsequent retrieval through electronic search, which is a generalized practice for the identification of these studies in the published literature[8]. However, this recovery is considered insufficient; about 35% of controlled clinical trials are not identified in an automated search for various reasons[9]. In general, the authors do not describe the research method clearly; the use of the descriptor "controlled clinical trial" is not frequent and other terms available to describe the design of a trial are not used exhaustively[9].
A classic example of an insufficient description is related to masking when it uses broad, ambiguous and inconsistent terms in addition to the infrequent explicit description of the blinding of participants and staff[10]; even though the descriptors "double-blind method" and "single-blind method" were among the first to be introduced as authorized medical descriptors. There is also evidence that most of the items required by CONSORT are reported in less than 50% of cases[11].
Such gaps in the description of clinical trials difficult to recover them by electronic searching and that make necessary the manual search for which the Cochrane organization promotes an international project. The manual search of controlled clinical trials consists of a "page by page" review in the title, abstract and patients/methods sections in each article of each journal number[9]. This method has not only become indispensable to recover the trials that are not obtained by electronic search, but it also corroborates that the title is not always enough; that the abstract is the section where keywords are most frequently located; and that reading the methods is usually necessary to ensure that the randomization method used has been correctly explained[9].
The literature reports interesting studies that, based on favorable results in the combined use of both search methods, assert that combining them is the ideal strategy. The diversity of the journals that have been studied, both from specialties and general medical journals, confirm that the inconsistent description of clinical trials is a generalized practice[12],[13],[14],[15],[16],[17],[18],[19]. For this reason, and given the importance of recovering published clinical trials and using them in the construction of evidence, this type of research is required on the journals that publish these studies.
Cuban medical journals, which contain an important part of national clinical trials, have gone through the manual search but their results have been described through quantitative indicators of scientific productivity[20],[21],[22]. In none of the cases, the results were compared to electronic search strategies; nor is there evidence of an analysis of the terminology used for the description of these studies.
The purpose of this paper is to compare the recovery capacity between manual search and multiple strategies of electronic search to locate clinical trials in Cuban medical journals, and to determine the terminology used in several sections of the article to describe the clinical trial.
Methods
A descriptive investigation was carried out. The manual search was conducted using the rapid localization method established by the Cochrane guide, which consists of the identification of keywords in the title, abstract, and methods[9]. The sample consisted of all the numbers published in the period 2000-2012 in Cuban Journal of Medicine, Cuban Journal of Tropical Medicine and Cuban Journal of Stomatology. These journals were selected because they are the most productive Cuban publications of clinical trials, according to the manual search files available at the National Center for Clinical Trials Coordinator, member of the Collaborating Center of the Ibero-American Cochrane Network in Cuba.
An electronic search was conducted through the Cumed database and the SciELO Cuba virtual library on January 4, 2018, to check if the clinical trials previously identified in the manual search were retrieved in the electronic search. Cumed is the Cuban medical bibliography database, which offers bibliographic references of works published in Cuba or abroad by Cuban authors[23]. SciELO Cuba is an electronic library that includes a selection of Cuban scientific journals in all areas of knowledge; the Telematic Health Network in Cuba/INFOMED develops it in collaboration with the Latin American and Caribbean Center for Information on Health Sciences of Brazil (BIREME), and it is part of the SciELO Regional project[24].
The Cumed search was done in two ways. The first one through the advanced form, using all the terms available in the index, by the field type of publication; the strategy was the following:
- "ENSAYO CLINICO" OR "ENSAYO CLINICO CONTROLADO" OR "ENSAYO CLINICO CONTROLADO A" OR "ENSAYO CLINICO FASE II" OR "ENSAYO CLINICO FASE III" OR "ENSAYO CONTROLADO ALEATORIO" [Type of publication].
The second search was made by journal title, using for each one, the free terms "essay or essays" in the words of the title; the strategies used were:
- "REV. CUBA. MED. TROP/2000,5" or "REV. CUBA. MED. TROP/2001,5" or "REV. CUBA. MED. TROP/2002,5" or "REV. CUBA. MED. TROP/2003,5" or "REV. CUBA. MED. TROP/2004,5" or "REV. CUBA. MED. TROP/2005,5" or "REV. CUBA. MED. TROP/2006,5" or "REV. CUBA. MED. TROP/2007,5" or "REV. CUBA. MED. TROP/2008,6" or "REV. CUBA. MED. TROP/2009,6" or "REV. CUBA. MED. TROP/2010,6" or "REV. CUBA. MED. TROP/2011,6" or "REV. CUBA. MED. TROP/2012,6" [Journal] and "ENSAYO" or "ENSAYOS" [Title Words]
- "REV. CUBA. MED/2000,39(1)" or "REV. CUBA. MED/2000,39(2)" or "REV. CUBA. MED/2000,39(3)" or "REV. CUBA. MED/2000,39(4)" or "REV. CUBA. MED/2002,41(1)" or "REV. CUBA. MED/2002,41(2)" or "REV. CUBA. MED/2002,41(3)" or "REV. CUBA. MED/2002,41(4)" or "REV. CUBA. MED/2002,41(5)" or "REV. CUBA. MED/2002,41(6)" or "REV. CUBA. MED/2003,42(1)" or "REV. CUBA. MED/2003,42(2)" or "REV. CUBA. MED/2003,42(3)" or "REV. CUBA. MED/2003,42(4)" or "REV. CUBA. MED/2003,42(4)" or "REV. CUBA. MED/2003,42(5)" or "REV. CUBA. MED/2003,42(6)" or "REV. CUBA. MED/2004,43(1)" or "REV. CUBA. MED/2004,43(2-3)" or "REV. CUBA. MED/2004,43(4)" or "REV. CUBA. MED/2004,43(5/6)" or "REV. CUBA. MED/2005,44(1-2)" or "REV. CUBA. MED/2005,44(3-4)" or "REV. CUBA. MED/2005,44(5-6)" or "REV. CUBA. MED/2006,45(1)" or "REV. CUBA. MED/2006,45(2)" or "REV. CUBA. MED/2006,45(3)" or "REV. CUBA. MED/2006,45(4)" or "REV. CUBA. MED/2007,46(1)" or "REV. CUBA. MED/2007,46(2)" or "REV. CUBA. MED/2007,46(3)" or "REV. CUBA. MED/2007,46(4)" or "REV. CUBA. MED/2008,47(1)" or "REV. CUBA. MED/2008,47(2)" or "REV. CUBA. MED/2008,47(3)" or "REV. CUBA. MED/2008,47(4)" or "REV. CUBA. MED/2009,48(1)" or "REV. CUBA. MED/2009,48(2)" or "REV. CUBA. MED/2009,48(3)" or "REV. CUBA. MED/2009,48(4)" or "REV. CUBA. MED/2010,49(1)" or "REV. CUBA. MED/2010,49(2)" or "REV. CUBA. MED/2010,49(3)" or "REV. CUBA. MED/2010,49(4)" or "REV. CUBA. MED/2011,50(1)" or "REV. CUBA. MED/2011,50(2)" or "REV. CUBA. MED/2011,50(3)" or "REV. CUBA. MED/2011,50(4)" or "REV. CUBA. MED/2012,51(1)" or "REV. CUBA. MED/2012,51(2)" or "REV. CUBA. MED/2012,51(3)" or "REV. CUBA. MED/2012,51(4)" [Journal] and "ENSAYO" or "ENSAYOS" [Title Words]
- "REV. CUBA. ESTOMATOL/2000,3" or "REV. CUBA. ESTOMATOL/2001,3" or "REV. CUBA. ESTOMATOL/2002,3" or "REV. CUBA. ESTOMATOL/2003,4" or "REV. CUBA. ESTOMATOL/2004,4" or "REV. CUBA. ESTOMATOL/2005,4" or "REV. CUBA. ESTOMATOL/2006,4" or "REV. CUBA. ESTOMATOL/2007,4" or "REV. CUBA. ESTOMATOL/2008,4" or "REV. CUBA. ESTOMATOL/2009,4" or "REV. CUBA. ESTOMATOL/2010,4" or "REV. CUBA. ESTOMATOL/2011,4" or "REV. CUBA. ESTOMATOL/2012,4" [Journal] and "ENSAYO" or "ENSAYOS" [Title Words]
For the search in SciELO Cuba (available at http://scielo.sld.cu/scielo.php) it was used the free form that offers up to three options of terms to combine. Ten terms were selected from the available ones and were combined through the "OR" operator in the "all indexes" field. The strategies in SciELO Cuba were the following:
- ENSAYOS CLINICOS [All indexes] OR ENSAYOS CLINICOS ALEATORIZADOS [All indexes] OR ENSAYOS CLINICOS CONTRA EL CÁNCER [All indexes]
- ENSAYOS CLINICOS CONTROLADOS [Todos los índices] OR ENSAYOS CLINICOS FASE I [Todos los índices] OR ENSAYOS CLINICOS FASE II [All indexes]
- ENSAYOS CLINICOS FASE III [Todos los índices] OR ENSAYOS CLÍNICOS MULTICÉNTRICOS [All indexes] OR ENSAYOS CLÍNICOS, INVESTIGACIÓN [All indexes]
- ENSAYOS CONTROLADOS ALEATORIOS [All indexes] In this database, a search was also carried out through the basic form using free terms in the title words. The strategy used was:
- ENSAYO OR ENSAYOS [Title Words]
For the analysis of the terminology the authors took as reference: the descriptors of thesaurus Descriptors in Health Sciences (DeCS)[4]; the terms proposed by the document "Identification of controlled clinical trials: Manual search guide of the Ibero-American Cochrane Center"[9] and some CONSORT criteria[6]. A data sheet was created in Excel organized by journals, by article, and by sections to register the terms. The descriptors and terms of reference, according to the source used, were the following:
DeCS: prospective studies, comparative study, double-blind method, single-blind method, and as descriptors of type of publication clinical trial, randomized controlled trial, multicenter study, clinical trial, phase I, clinical trial, phase II, clinical trial, phase III, clinical trial, phase IV, controlled clinical trial, cross-over studies y pragmatic clinical trial[4].
Cochrane Guide: random assignment (randomization), quasi-random assignment (quasi-random), controlled trial, blinding or masking, cross-over trial, open clinical trial, prospective study, retrospective study (as excluding criterion), control group, placebo, randomized selection and allocation randomized[9].
Consort: Although it does not require specific terms, it requires that a trial should be identified as such in the title section (eg, randomized trial); methods section demand specifying the type of design (for example parallel, factorial); requests specific information on randomization (eg, type of randomization, method to generate randomization sequence), and masking[6].
The selection of general descriptors and the exact terms, or other related ones, was taken into consideration if they made it possible to identify a clinical trial by an item that is part of the design. We considered, therefore, prospective study because it indicates that there has been previous planning of the interventions and assignment of the subjects before the start of the data collection. We used the terms comparative or groups because two or more interventions are compared to each other, which is also related to being controlled. We used randomization because it makes explicit whether the method of chance was used in the allocation of participants to the groups that are compared.
The mention of the study phase was also identified as being a distinctive feature of the clinical trial, as well as the terms related to blinding that imply masking, at different levels, the assignment to each treatment group. We also looked for any other specific design term like parallel, factorial, pragmatic, among others. Finally, other more general concepts like multicenter study were used, since more than one health institution usually participates in the evaluation, treatment, and follow-up of patients and is a common qualifier in this type of research; and placebo for referring to an inactive substance that is usually administered to patients in a trial as a comparison intervention.
Results
In the manual search, 50 trials were identified, 11 in the Cuban Journal of Tropical Medicine, 14 in the Cuban Journal of Medicine and 25 in the Cuban Journal of Stomatology. Taking into account the poor description of the studies verified in the identification process, the studies in which test condition was explicit or was evident due to the fulfillment of some of the criteria, and which they classified as possible trials, were considered according to the criteria of the Cochrane manual search guide[9].
The electronic search in Cumed retrieved four previously identified trials in the manual search. With the strategy by specific free terms available in the publication type index, 79 records were recovered, of which 53 were clinical trials published in national and foreign sources (67.9%), and none of the journals studied. The search strategy for journals, with general free terms available in the title, returned 12 records, of which four were clinical trials of the journals and periods analyzed.
The electronic search in SciELO Cuba identified five of the trials of the manual search. With the strategies by specific free terms available in all the indices, 77 records were obtained; of these, 19 clinical trials and four were manually identified. The strategy by general free terms, available in the title, recovered 220 results, of which 11 were clinical trials and five of the journals studied.
The three electronic searches that identified trials obtained by the manual procedure recovered six studies that represented 12% of the total identified by that method. Three trials were from the Cuban Journal of Tropical Medicine (3/11- 27.2%) and three from the Cuban Journal of Medicine (3/14- 21.4%). The three strategies recovered only two of them. Neither of the two databases retrieved an assay that had not been previously identified by the manual search.
Of the six trials identified in the electronic search, four included significant terms in all sections. Five of them contained them in the title (four said a clinical trial and one included a therapeutic trial). Five included terms in the keywords; the study that did not have them in this section did include a clinical trial in the title and abstract. Except for the title section, in the rest of the sections of the six articles, clinical trials in the singular and clinical trials or trials in the plural, linked or not to another concept, were indistinctly used.
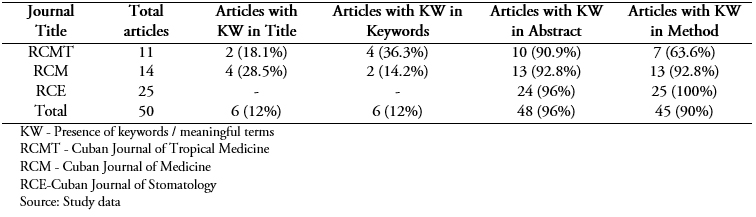
Table 1 summarizes the total number of articles per journal and the number of articles that contained significant terms in each of the sections analyzed.
 Full size
Full size The findings were similar in the three journals: the title and the keywords were the least descriptive sections; the abstract and methods were the most descriptive. No journal had a high proportion of articles with significant terms in all sections.
The analysis of the terminology used in each article, by sections and by the occurrence of terms (descriptors, keywords, concepts, terms, and significant phrases), allowed a qualitative analysis of the description of the trials. The following tables (2, 3 and 4) summarize the use of the most specific terms to refer this type of study (clinical trial, controlled, randomized, single or double-blind, phase, therapeutic trial, registration code). We excluded from them, but not from the analysis, other terms that, although they may provide a suggestion of a clinical trial, are not confirmatory on their own since they apply to other types of study (comparative study, study groups, placebo, prospective study, experimental design) as well.
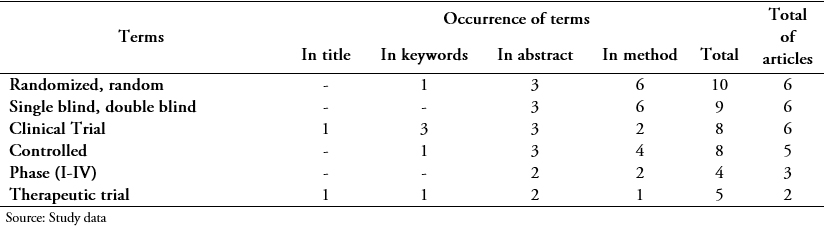
Table 2 shows the analysis of the results for the Cuban Journal of Tropical Medicine where all the reports included significant words in at least one section. The first column contains the number of articles where the terms appear, and the successive columns show the breakdown of occurrences by sections.
 Full size
Full size Of the 11 articles, two titles (18.1%) had relevant terms (clinical trial, therapeutic trial), and both also had it in the abstract; one of them, in the keywords and no case was repeated trial or clinical trial in the method. Of the four articles that included significant terms in the keywords (36.3%), all said essays or essays.
Ten reports contained keywords in the abstract (90.9%); four included them only in that section and two of them related to a single concept (comparison). One used the phrase “the method was compared” and the other used “comparative study”. In the other two, words like control group and placebo were found in one of them; and placebo, clinical trials, phase I-phase II in the other. In the methods section, seven articles included significant terms (63.6%); the remaining four did not use them in the title or the keywords, only in the abstract.
The terms with the highest occurrence were randomization (10), blinding (9) and clinical trial (8); all were used in six articles (54.5%). It highlighted the use of clinical trial in a title and the keywords of three articles. The lack of consistency between the sections was evident because not all the articles repeated the terms used in each one. For example, of the three trials that used the terms clinical trial or clinical trials in keywords, only one used it in the method.
The trial phase was used in three reports and in no case in the title or keywords. A therapeutic trial was used in two reports (five occurrences), and indistinctly in any of the sections while the registration code was not used.
The less specific words used were related to comparative study and study groups (four articles), placebo (three) and experimental design (one). Others as an open/multicentre/unicentric trial/study were not used.
Of the three articles of this journal that were retrieved in the electronic search, only one had significant terms in all the sections, and although it used few words, these were very specific, such as a therapeutic trial in the title, therapeutic trial and comparatively in the abstract, and in the method comparative study and study groups. There was a study that did not include words in the title but was explicit in the keywords, the abstract and the method with the terms clinical trials, randomized, controlled, double-blind. Another, which did not contain them in the keywords, was sufficiently descriptive in the other sections using a clinical trial, controlled, randomized, double-blind. One of the studies referred to the random concept, but it was not related to the design of the trial (random table).
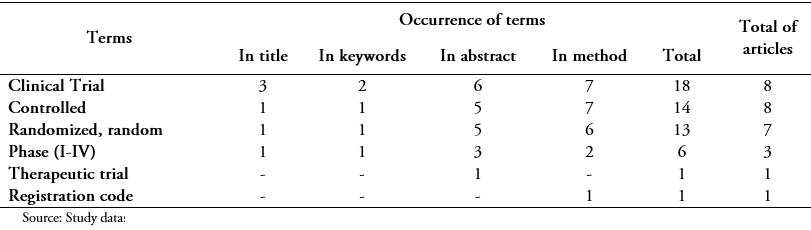
In the case of the Cuban Journal of Medicine, which reported 14 trials, the behavior was similar. Table 3 shows the frequency of significant terms used in this journal.
 Full size
Full size The 14 articles of this journal included significant words in some section. Four studies used them in the title (28.5%); in three of them, a clinical trial was used and in one comparison. The latter did not include any term in the keywords but was more explicit in the abstract with a prospective trial and in the method where it used a prospective study and a clinical trial. Two of these articles contained the term clinical trial or trial in all sections.
Two reports included significant terms in the keywords (14.2%); one of them randomized controlled trials and the other phase I clinical trials/methods. They both mentioned the word essay in all sections and, although they did not always use the clinical adjective, they rated it with others as random, controlled.
The abstract was the most descriptive section; 92.8% of the studies included some significant relevant word (13/14). Two articles contained it only in this section and related to a single concept (comparative study, comparison), the sentences were as ambiguous as both methods were compared and the results were compared. There was a trial that included only terms in the method, but with a combination resulting exhaustive: controlled and randomized clinical trial and two comparative groups.
The terms most used were related to clinical and controlled trials in eight studies each (57.1%), followed by randomization in seven (50%). In this review, the only study that reported a code from the Cuban Public Registry of Clinical Trials (RPCEC 00000083) was published and published in 2012, although it was found that the code was erroneous.
Words related to the study phase and the therapeutic trial were scarcely used and no article described methods related to masking, thereby blind, single or double blind terms were not used. One of the articles used masking as a subtitle to explain that this was not done.
Regarding the use of ambiguous terms, comparative study and study groups were used (five studies), prospective, multicentric/unicentric study, open study/trial (four) and experimental design (one); the term placebo was not used.
The phrases pilot study and longitudinal study were also identified, and in any case, it coincided with the use of clinical trial. However, they did use other descriptive terms of the type of study; in the case of the longitudinal study it also specified open, prospective, randomized and in the case of the pilot study he described it as open, controlled, randomized.
One of the electronic search strategies recovered the only three studies that included a clinical trial in the title; two of them had significant terms in all the sections, and the only one that did not contain them in the keywords used clinical trial, open phase II study, multi-center in the other sections.
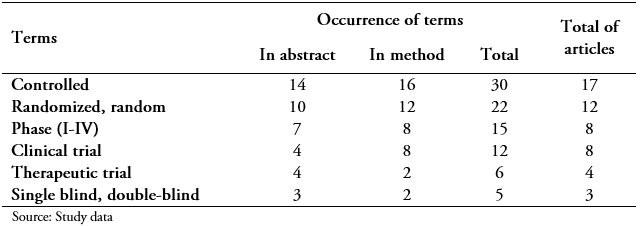
Table 4 shows the occurrence of terms in the Cuban Journal of Stomatology, which reported the largest number of clinical trial reports with 25.
 Full size
Full size None of the reports had significant terms in the title or in the keywords; 94.1% (24/25) used them in the abstract and 100% (25/25) in the method. In the abstract section, 18 studies used words related to more than one concept; six referred to only one, and an article did not use meaningful words. Also in the methods section, 19 studies described more than one concept. Eight trials addressed a single concept in the abstract or method; four of them repeated the same word in both sections (two used groups, one used longitudinal prospective study and another blind); and the article, which did not have significant terms in the abstract, referred only to groups in the method.
The most used significant words were related to controlled, used in 17 trials (68%), with a notable difference with the closest ones as randomized (12- 48%), phase and clinical trial (8- 32%); the rest were present in less than 20% of the trials (therapeutic trial, blinding) while the registration code was not used.
Among the ambiguous terms, there was a high frequency of use of study groups (17/25- 68%). Four investigations used experimental study / experimental group; one used, also, the term clinical trial, and the rest was sufficiently explicit with the use of qualifiers as randomized or controlled, and treatment groups. Less used were prospective study, comparative study (two) and multicenter/unicentric (one).
About the comparison concept, it was mistakenly used: the drug was compared, since in a clinical trial the groups must be comparable and then the results that evaluate the efficacy or effectiveness of the intervention. An article contained significant terms only in the method, and these did not facilitate its identification; the terms used were the sample was divided into two groups, group 1, and group 2.
Contradictory was the fact that one of the reports only used the blind term in the abstract and in the method; the study compared two techniques for the installation of previously installed single implants where it is only specified that the professional who applied the intervention was blind to the assembly conditions.
In an integrated analysis of the use of terms in the total number of trials, it turned out that the most specific ones to identify one trial were little used: clinical trial (22- 44%) and phase I-IV (14- 28%). There were used, in more than half of the trials, others related to controlled (30- 60%) and randomized (25-50%), in addition to those concerning study groups (26- 52%).
The use of specific words related to blinding (9- 18%), therapeutic trial (7- 14%) and the registration code was not representative. Neither was the use of others less precise as a comparative study (11-22%), prospective study (8- 16%), experimental design (6- 12%) and open trial/study (4- 8%). They included some significant term; one explicit therapeutic trial, another used comparison, and four contained the term clinical trial (7.8%). General descriptors related to the study characteristics such as evaluation studies, validation studies, and clinical study were not used.
Discussion
The results of the search by both methods corroborated that the electronic search was not sufficient to identify clinical trials. The coincidence of the registries identified in both was almost null, and the manual search showed a greater capacity of retrieval, thereby considered superior. The fact that electronic search did not provide any new study to those identified by manual search was similar to those reported in the literature that refers very low or no retrieval rates of the first method compared to the second. Such is the case of two studies that, when comparing both search methods, one electronically recovered 2% of the total of identified trials (2/103)[19] and the other 4% (32/174)[12].
On the other hand, Hopewell and collaborators, in a comparison between manual and electronic search in MEDLINE, to identify trials in 22 medical journals in the United Kingdom, demonstrated the indispensability of the first method. Of 462 trials indexed in MEDLINE, 117 were identified only by manual search (25%)[12]. A similar study using a cluster design to identify clinical trials in a group of pre-selected journals also showed that 25% of the studies were identified only by manual search[17].
These results confirm the findings of the study by Suárez[14] who concluded that to carry out an exhaustive search it is necessary to combine the manual and electronic methods, adding that for the latter it is necessary to use two or more databases. This study, which identified 4111 trials by electronic search, demonstrated a superior performance of EMBASE over MEDLINE (85% vs. 73%) to identify trials in a group of pre-selected journals and topics.
The above statement is valid also for this study, taking into account the discrepancies in the results obtained in Cumed and in SciELO Cuba. However, in both resources, the search strategies were specific and, in theory, highly sensitive. Terms related to the clinical trial were used as a publication type, but a large number of records were recovered that addressed the clinical trial as an issue due to inappropriate indexing.
This behavior was contradictory with the wide variety of authorized general medical descriptors that are available to describe a clinical trial. This is currently represented under the publication type category; in one case as a descriptor in the clinical study subcategory and in the subcategories validation studies and evaluation studies. The diversity of available descriptors corroborates that, since the 1960s, authors, editors, arbitrators, and indexers have a sufficient number of pre-established terms to describe the content of these studies under a homogeneous terminology[4],[5].
General descriptors such as a prospective study and a comparative study, as well as a double-blind method were introduced between 1965 and 1977; while single-blind method and type-of-publication qualifiers such as clinical trial, randomized controlled clinical trial, multicenter study, phase I clinical trial, phase II clinical trial, phase III clinical trial, phase IV clinical trial, controlled clinical trial, cross-over studies were added between 1990 and 1995 (although the qualifier specification was eliminated in 2008); and pragmatic clinical trial, in 2014. The descriptor "clinical trial as a subject" was incorporated in 2008 to identify works on the subject and not the results report of a particular trial[4],[5].
Unlike the researches related above, this article analyzed the terms used to describe the study design. The most descriptive section was the abstract, which corresponds to what the Cochrane manual search guide describes[9]. The presence of keywords in the title and in the section of keywords had a very low frequency; precisely the fields in which the search process is most focused.
While the description in the title and keywords of the journal of dentistry was null, those of medicine and tropical medicine had occurrences, although minimal. A more complete and consistent description of the abstract and the method predominated in the stomatology trials, although this journal showed the lowest frequency of use of the term clinical trial. In the other two publications, the descriptive elements were better distributed among the different sections.
Although the title and keywords sections were the least descriptive, in the Cuban Journal of Tropical Medicine and in the Cuban Journal of Medicine, precise terms and phrases were used that facilitated the recovery. All four trials that included a clinical trial in the title were retrieved; one of them was the only one that used a clinical trial, controlled and randomized in that section, among 30 that claimed they had the first condition (controlled) and 25 the second (randomized). As in a study conducted to identify clinical trials by conglomerates, which reported around 50% of trials identified by these terms in the title and abstract[17], this result confirms the importance of a sufficiently descriptive title. The scarce use of these terms in the title corroborated that the recommendations of the CONSORT statement were not taken into account[6]; none of the Cuban journals indicated this regulation, arising since 1996, in its instructions.
On the other hand, the inclusion of specific terms in the keywords was not sufficient for the success of the electronic search. Two trials that were not retrieved included therapeutic trials (Revista Cubana de Medicina Tropical) and a randomized, controlled clinical trial (Revista Cubana de Medicina) as keywords.
80% of the studies referred to terms related to more than one concept (40/50) but there was an inconsistency between the sections, except the Cuban Journal of Stomatology, since descriptive terms were not repeated in all cases. Two essays in the Cuban Journal of Tropical Medicine and two others in the Cuban Journal of Medicine referred to a single concept, more ambiguous, related to comparison; and another six from the Cuban Journal of Stomatology linked to masking (one), prospective study (one) and study groups (four). The latter used terms such as groups, group A and group B, group 1 and group 2 or two groups, without specifying what type of group it was.
Regarding the concept of groups, other studies used words that are more specific as a control group for one, and for the other experimental group, treatment group or group "name of the intervention". In general, a high level of use of this concept was appreciated, even over clinical trial, randomized and phase. The trial phase, which is a distinctive element and could facilitate recovery, was underutilized; the only study that used it in the title was from the Cuban Journal of Tropical Medicine, and it was recovered in the electronic search. One of the studies above highlighted that 25% of the trials could have been identified by the reported randomization units, but these were not susceptible to electronic search[17].
Non-compliance with the prospective registration of a trial as a precondition for publication in a scientific journal is the responsibility of both authors and editors. The only trial supposedly registered had an erroneous code. The article refers to an essay on the efficacy of microdoses of captopril in arterial hypertension, while the registry corresponds to a study of leukocim in oncohematological patients. This shows that the researchers did not comply as required by the Helsinki Declaration nor did the editors verify the veracity of the primary data. This aspect, together with the low use of the term clinical trial in the title, corroborated that the recommendations of the CONSORT statement were not taken into account; none of the magazines alludes to the use of this regulation that has emerged since 1996.
It was significant that, in the Cuban Journal of Stomatology, three articles referred to randomly selected samples, which was confusing to identify the clinical trial. This is because the chance is used to assign the participants to each group and not for their selection.
The previous results confirm the affirmation of Taljaard and collaborators, when they pointed out that the variability in the terminology used is what turns the electronic search into a challenge, to which the use of ambiguous terms can be combined. Another important consideration of this study was the increase in the proportion of clearly identified trials, between the periods 2000-2003 and 2004-2007 from 28% to 60%17. This trend, which could mean an increase in the level of knowledge and improvements in the practices of the professionals involved, contrasts with the present investigation where the trials identified by the electronic search are concentrated between 2000 and 2003.
A study to identify trials in dermatology journals in Spanish (Latin American and Spanish) also showed that manual search was more effective than electronics. The authors said that probably, in this case, it was due to the low sensitivity of MEDLINE and EMBASE for the detection of descriptors or terms in Spanish. Something that did not happen in the present study because the information resources used and the articles are in the Spanish language[18].
In general, authorized descriptors were not used as they were conceived, but synonyms and terms related to the concepts they define were used. The performance of the electronic search was not always proportional to the completeness of the indexing or the specificity of the search.
Final considerations
The development of electronic search strategies that, even with specific terms, were not sensitive to identify the trials; the presence of studies that did not recover, with an index similar to those that were recovered implies multiple causes in the low rates of recovery. These could be divided between the poor description by the authors, insufficient indexing during processing to enter the records in the databases, and inconsistencies of search algorithms and retrieval of these, which do not always conceive all relationships between the terms used.
Underutilization of authorized descriptors is then a general practice among authors, reviewers, editors, and indexers and hinders recovery by electronic search and identification by manual search. Complementing both methods is a strategy to achieve greater performance in the search. However, anticipated actions that promote shared responsibility among the actors involved in the process are required.
It is necessary to strengthen the training of researchers, members of the editorial committees and information professionals who are in charge of indexing these studies in the databases. It is also necessary to extend this type of study to other journals of medical specialties to determine professional practices in this regard, in addition to conducting other research on the adherence of Cuban clinical trial reports to established international standards in order to improve communication, the recovery, and the use of the results of this type of study.
Notes
Annexes
Author contributions
The authors declare that they both fulfil the four authorship criteria of the ICMJE (International Committee of Medical Journal Editors) and have contributed to the conceptualization, design, writing and critical revision of the contents of this article.
Conflict of interest statement
Both authors have completed the ICMJE declaration of conflicts of interest statement, and declare that they do not have any competing interests with the contents of this article.
Funding
There was no funding for this work.
From the editor
The Journal declares that this is a translated version of the original article, which was submitted in Spanish, and was lightly copyedited by the journal editor before its publication.

