Case reports
← vista completaPublished on June 19, 2019 | http://doi.org/10.5867/medwave.2019.05.7655
A case report of tuberculous chylothorax
Reporte de un caso de quilotórax tuberculoso
Abstract
Tuberculous chylothorax is a rare infectious disease that occurs when the thoracic duct is obstructed. Treatment is directed to the tuberculosis infection. A 55-year-old male, driver, born in Trujillo (Peru) is admitted to the emergency department with increasing dyspnea and a 5-day dry cough. The physical examination revealed vocal fremitus, dullness to percussion, and a vesicular murmur that was decreased on the lower 2/3 of the left hemithorax. The X-ray and the thoracic ultrasound revealed significant left pleural effusion. The thoracocentesis drained fluid identified as chylothorax. Subsequently, a thoracic tube was placed, with a decrease in pleural fluid volume and later normalization of the cytochemical changes. Diagnostic video bronchoscopy was performed with a bronchoalveolar aspirate, revealing acid-fast bacilli. The patient received antituberculosis treatment with a favorable outcome. Tuberculous chylothorax is an important cause of chylothorax to be considered in endemic areas of tuberculosis. Proper treatment of the infection leads to resolution of the disease.
|
Key ideas
|
Introduction
Chylothorax is a type of pleural effusion caused by the accumulation of chyle, a fat-enriched liquid secreted by intestinal cells and collected and transported by the thoracic duct to systemic circulation. It occurs as a result of damage to or blockage of the thoracic duct[1],[2],[3].
Tuberculous chylothorax is diagnosed through microbiological isolation of Mycobacterium tuberculosis in the lung parenchyma, pleura, or extrathoracic areas[4]. Imaging techniques usually show pulmonary infiltrates and mediastinal lymphadenopathy in addition to pleural effusion, but the absence of radiologically abnormal lung parenchyma does not rule out the diagnosis[5].
This report describes a case of tuberculous chylothorax, an interesting clinical condition due to its rare occurrence and the diagnostic challenges it poses.
Clinical case
A 55-year-old male patient, a driver from Trujillo, Peru, presented at the emergency department (ED) of the Regional Teaching Hospital of Trujillo (Hospital Regional Docente de Trujillo) complaining of progressive shortness of breath (dyspnea) and five days of sporadic non-productive (dry) cough. Three days before admission he began experiencing throbbing, non-radiating pain of moderate intensity in the left thoracic region and dyspnea with moderate exertion. One day before admission the symptoms worsened, including dyspnea while resting. The patient had no medical or surgical history.
Upon physical examination the patient was afebrile, with no tachypnea, and an oxygen saturation of 93% while breathing ambient air. Other vital signs were stable, with no signs of respiratory distress, and the patient appeared to be sufficiently nourished and hydrated. In the left hemithorax the vocal fremitus was diminished, with dullness on percussion and reduced vesicular murmur in the lower two-thirds. The physical exam was otherwise unremarkable.
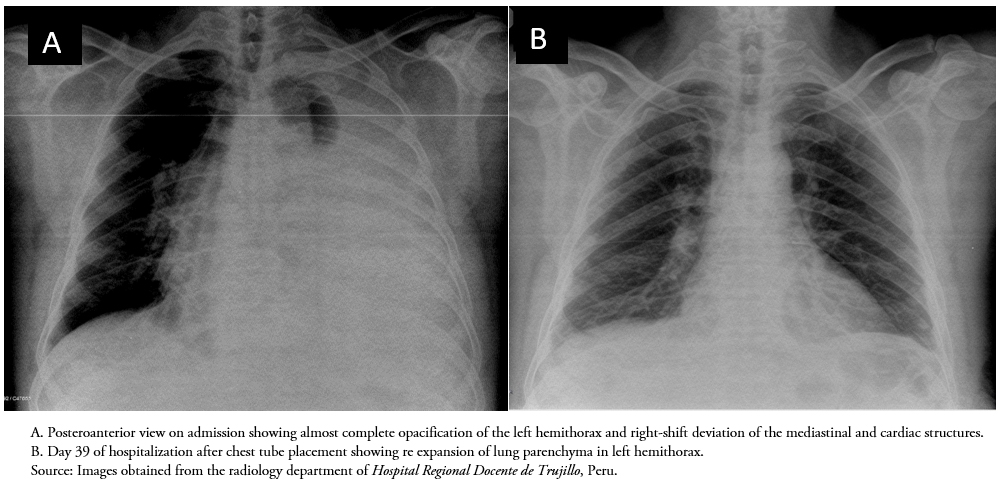
The initial chest X-ray (Figure 1A) and thoracic ultrasound showed a large left pleural effusion, so a diagnostic thoracentesis was carried out, draining 2000 ml of milky fluid (Figure 2A), and the patient was hospitalized in the pulmonary department for further studies and treatment. Due to the rapid reaccumulation of chylothorax, on the fifth day of hospitalization the patient underwent insertion of a pleural drainage tube, which later became obstructed. Given the large volume of pleural fluid drained through the tube during the first few weeks, a diagnostic thoracoscopy with possible thoracic duct ligation was recommended. The patient declined, so serial therapeutic thoracentesis (removing an average of 1500–3500 ml of fluid per procedure ) was required. With the obstruction a new chest tube was inserted for an additional 18 days, during which time there was a decrease in pleural drainage of 20-30 ml per procedure and a change in the appearance of the chylothorax fluid from its initial milky appearance to a straw yellow color (Figure 2B).
The cytochemical characteristics of the initial pleural fluid are shown in Table 1. The blood culture, sputum culture, and pleural fluid culture showed no bacterial development. Results were also negative for antibodies for human T-cell lymphotropic virus, the ELISA test for human immunodeficiency virus (HIV), and serologic markers for viral hepatitis. Table 2 shows the procedures and results of the laboratory tests requested for this case upon admission and throughout hospitalization.
The selected treatment of the chylothorax was 0.5 mg of octreotide subcutaneously every eight hours for 28 days. This protocol was administered irregularly for economic reasons (the hospital did not provide the medication because the patient was not covered by Peru’s Comprehensive Health Insurance (Seguro Integral de Salud or SIS); therefore, the patient had to pay for the medicine). The patient also received 10 days of parenteral nutrition (electrolytes, amino acids, proteins and lipids (medium-chain triglycerides- SMOFlipid® 20%)) by central venous catheter. Subsequently, a special elemental diet (Alitraq®) (300 ml per day, divided into three doses every 8 hours orally, increased every 72 hours according to tolerance and drainage), was added for 34 days. A pancreatic enzyme capsule (CREON® 2500) was also given (every 12 hours orally for 22 days).
After achieving a significant reduction in the production of chylothorax, to obtain a better view of pulmonary and mediastinal structures, on the 15th day of hospitalization the patient underwent a thoracic tomography with contrast, which showed a small posterobasal pleural effusion with heterogeneous areas of pulmonary consolidation in the lower left lobe and slight thickening of the peribronchiovascular interstitium in both hemithorax (Figure 3). No mediastinal lymphadenopathies were found.
On the 39th day of hospitalization the patient developed a fever. A flexible fiberoptic bronchoscopy with a bronchial-alveolar aspirate was carried out to detect any pulmonary infectious causes of the fever. The results showed nonspecific inflammatory changes in the upper segment of the left lower lobe, which, like its lateral segment, is recognized as an aerobic environment for optimal growing of M. tuberculosis bacilli. The result of the bronchial-alveolar aspirate showed the presence of a paucibacillary sample (1-3 acid-fast bacilli).
Based on the bacteriological confirmation of the presence of Koch's bacillus as the infectious agent, the patient was diagnosed with chylothorax caused by pulmonary tuberculosis and started on daily therapy with isoniazid (300 mg), rifampin (600 mg), ethambutol (1200 mg), and pyrazinamide (1500 mg). After four days of treatment the patient’s clinical status improved significantly and the pleural effusion was controlled, as shown in the chest X-ray in Figure 1B). He remains stable, at home, and is being followed at the hospital’s outpatient pulmonary clinic.
 Full size
Full size  Full size
Full size 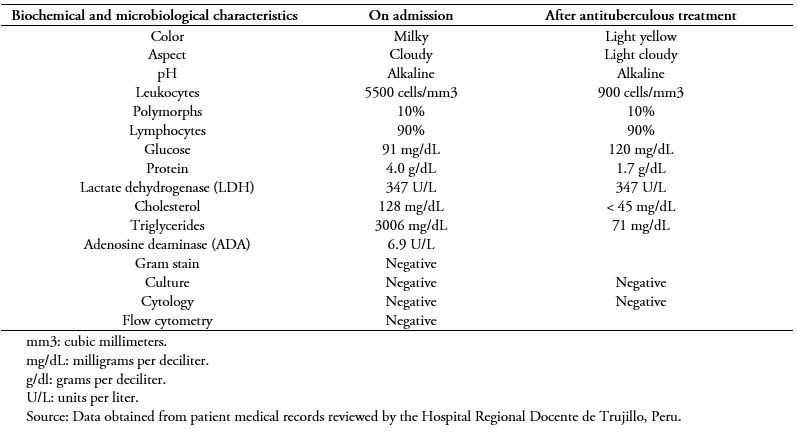 Full size
Full size 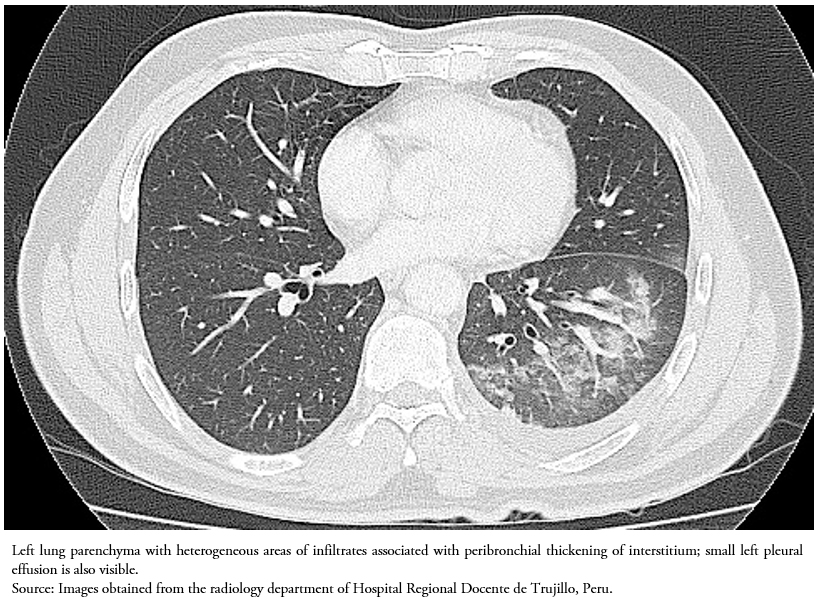 Full size
Full size 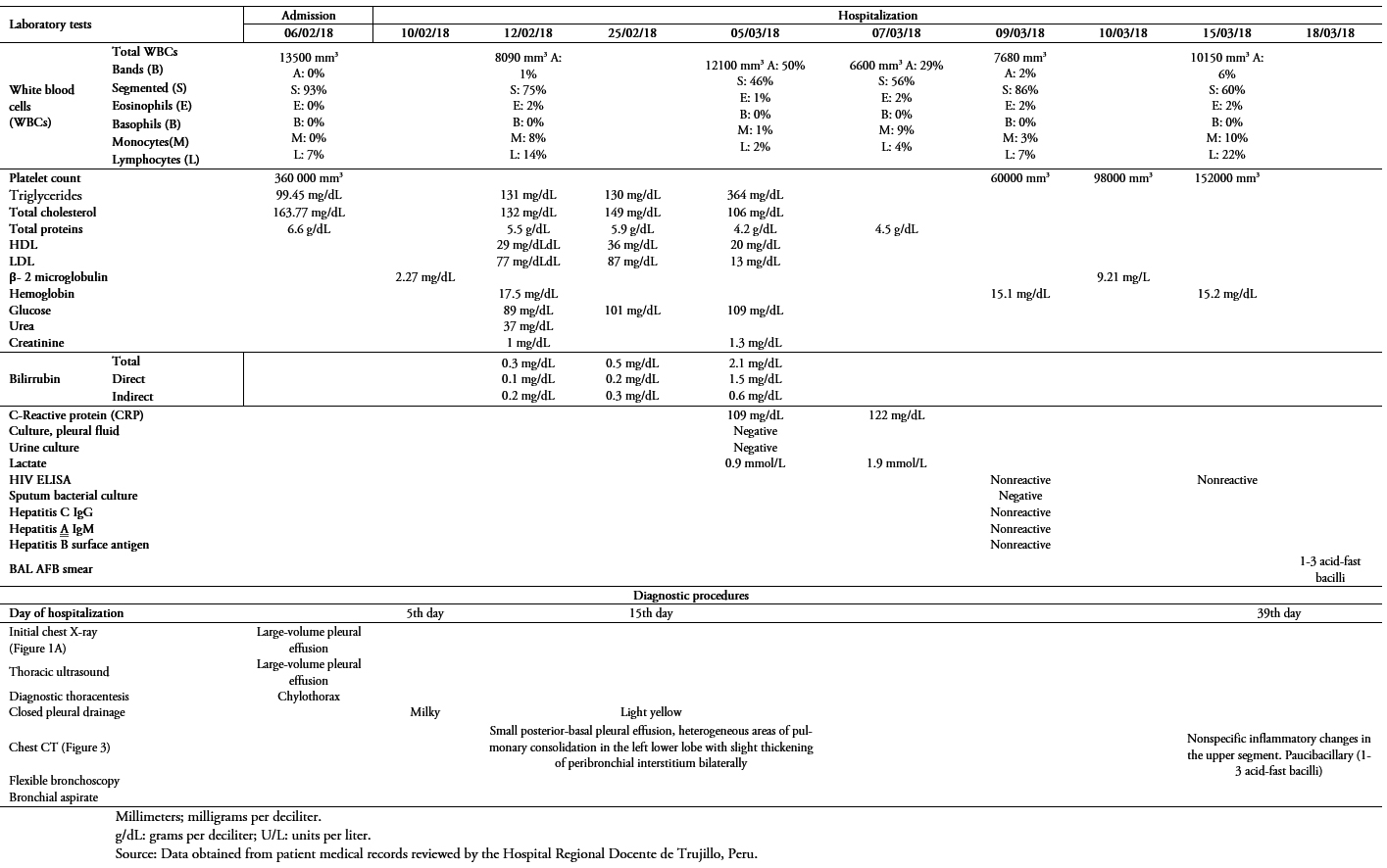 Full size
Full size Discussion
Chylotorax is a rare disease caused by disruption of the thoracic duct and direct accumulation of chyle in the pleural cavity, or by chylous ascites crossing the diaphragm into the pleural space. It can present unilateral or bilaterally depending on the level of lesion to the thoracic duct[5]. The typical fluid produced by this condition is a lymphocytic exudate with low concentration of lactate dehydrogenase[6] and bacteriostatic characteristics.
The most frequent cause of chylothorax is trauma, either accidental or iatrogenic. Of the non-traumatic causes, neoplasms, especially lymphoma, are the most common[1],[2],[7]. These etiologies were ruled out in our patient. The etiology and need for hospitalization will depend on the type of population and hospital facilities[8]. In a retrospective study by Doerr et al.[9] in a tertiary hospital in the United States that identified 203 patients with chylothorax, traumatic etiology was more common and no cases were due to tuberculosis[9],[10]. In another retrospective study, by Cortes-Telles et al.[8] in Mexico, 60% of chylothorax cases identified were non-traumatic and none were due to tuberculosis[8]. Multiple studies confirm that tuberculous chylothorax is extremely rare[5],[10],[11],[12], as is mortality attributed to it (6%)[4].
In Peru there are few reported cases of chylothorax. Due to our high prevalence and incidence of tuberculosis, diagnostic evaluation of chylothorax etiology should include pulmonary tuberculosis as it can produce a milky pleural fluid composed mainly of cholesterol called “pseudochylothorax”[10]. Few publications have described the association of tuberculosis with chylothorax, and the majority of reported cases are those that occur in association with direct trauma to the thoracic duct or due to pulmonary infiltration of neoplastic diseases[10].
The clinical case reported here had a non-traumatic etiology, without extensive pulmonary disease, but various diagnostic studies carried out during the hospitalization period led to confirmation of the presence of acid-alcohol–resistant bacilli in the BAL. It has been reported that in the majority of chylothorax cases associated with tuberculosis (72%) the two conditions are diagnosed simultaneously[4] rather than sequentially as in our case. The identified risk factor in our case was the patient’s occupation as a driver for patients coming to the hospital. As shown by this case it is important to consider tuberculosis in patients with chylothorax in endemic areas like Peru[13] or other endemic areas in Latin America.
Non-traumatic chylothorax has an insidious initial presentation[2] and varies according to the volume and velocity of fluid accumulation. For example, small-volume or early chylothorax is clinically silent, whereas large-volume or rapid-onset chylothorax can cause cough, shortness of breath, chest pain, and hypovolemia, due to the space-occupying effect[13]. Tuberculous chylothorax presents with constitutional symptoms (85%), dyspnea (60%), and cough (55%)[4],[14].
Radiologically, unilateral chylothorax appears as damage to the thoracic duct. The thoracic duct starts from the abdominal lymphatic ducts in the peritoneal cavity that merge at the posterior of the aorta below the diaphragm. From here the duct follows the direction of the aorta above the diaphragm to the right of the thoracic vertebrae and at the level of the third or fourth thoracic vertebrae turns to the left hemithorax, goes behind the esophagus[15], and drains into the left subclavian vein. Therefore, a lesion or obstruction above the level of the fourth thoracic vertebra will cause a left-sided chylothorax. In our patient the chest CT did not show an obstruction at this level.
It has been reported that tuberculous chylothorax is predominantly on the right side (45%) or bilateral (32%), and most commonly occurs with no lung abnormalities (51%). Mediastinal lymphadenopathy as well as the absence of mediastinal adenopathies have also been associated with this condition (46% and 29% respectively). In our case, the CT of the thorax showed posterobasal pleural effusion with heterogeneous areas of pulmonary infiltrates and no mediastinal lymphadenopathy.
Although the pathogenesis of non-traumatic chylothorax is not known with certainty, it is believed to be associated with compression of the cisterna chyli and thoracic duct due to enlargement of the mediastinal and hilar lymph nodes or from the opening of collateral anastomosis between the thoracic duct, azygos system, and intercostal veins. The compression caused by pressure from the lymphatic ganglia results in increased filtration of chyle into the pleural cavity[16],[17],[18],[19],[20],[21],[22],[23]. The CT for our patient showed bronchioalveolar abnormalities but not ganglionic ones, other than the chlothorax itself. Although we do not know how this patient’s pleural effusion occurred, we suspect that the thoracic duct and or lymphatic channels were directly infected with M. tuberculosis, based on evidence of thoracic and pulmonary disease[5],[23].
The diagnosis of chylothorax is not difficult. The appearance of a milky-looking fluid during thoracentesis should generate clinical suspicion. Differentiation with empyema is usually evident due to patient history of an infectious condition and the cytochemical characteristics of the pleural fluid. With pseudochylothorax there may be difficulties in the differential diagnosis; in this case the triglyceride level in the pleural fluid should be measured and anything higher than 110 mg/dL considered indicative of chylothorax. If the diagnosis is still not clear, lipoproteins can be measured and the presence of chylomicrons can be evaluated in order to confirm it[14].
In a publication by Gotuzzo the author states that tuberculosis is associated with chylothorax and pseudochylothorax[10]. This latter condition is defined as chronic pleural effusion rich in cholesterol[4]. Traditional methods for diagnosis of pleural tuberculosis have a poor sensitivity: acid-fast smears are frequently negative; pleural fluid cultures and “blind” pleural biopsies are only positive in 17% of cases; and pleural levels of adenosine deaminase (a leukocyte enzyme with reasonable sensitivity and specificity in highly endemic areas of tuberculosis) are only positive in 40% of cases[10]. However, a study published in 2018[24] shows that 95% of cultures of biopsy samples from thoracoscopy were positive for tuberculosis. This is confirmed in cases of tuberculous chylothorax. Pleural fluid acid-fast smears and cultures were positive in 14% and 24% respectively. Most confirmed cases of tuberculous chylothorax were established using lymph node biopsy (63%)[4]. In our case, the etiology of chylothorax was confirmed by acid-fast smear in BAL, which has been reported to have a low rate of positivity (27%) in these cases[4]. Our patient presented normal hematology and immunology in laboratory tests. The cytochemical study of the pleural fluid showed a lymphocytic exudate characteristic of chylothorax (cloudy and milky in color and with elevated triglyceride levels on admission, which decreased during therapy). Pleural fluid cultures were negative.
Management of non-traumatic chylothorax is mainly aimed at decreasing the production of chyle, and includes drainage of it, in cases of a symptomatic large pleural effusion. In general, there are two ways to treat chylothorax: surgically, and using conservative medical methods. Surgery entails ligation of the thoracic duct through thoracoscopy. Conservative medical treatment, for patients who are not surgical candidates, includes embolization of the thoracic duct, chemical pleurodesis, use of chronic pleural catheters, low-fat nutrition, and octreotide[25],[26],[27],[28],[29],[30],[31]. Our patient received conservative medical therapy, from admission through hospitalization, that included serial drainage of chylothorax using pleural drainage tubes. After starting antituberculous therapy the patient had a good clinical response, with decreased volume of pleural fluid drainage and normalization of its chemical characteristics versus those observed on admission.
Conservative medical treatment should also include daily replacement of albumin, total protein, lymphocyte, and electrolyte losses to prevent malnutrition and immunodeficiency[2]. Likewise, the diet should be modified with medium-chain triglycerides as the main lipid intake or the use of parenteral nutrition to limit chyle production and ensure an adequate replacement of lost proteins and nutrients[25]. Another therapeutic option is the use of octreotide, an analog of somatostatin with variable efficacy that is used to decrease the drainage of chyle. Our patient received and octreotide and, after insertion of a central venous catheter, parenteral nutrition (electrolytes, amino acids, proteins and lipids (medium chain triglycerides - SMOFlipid® 20%)). After 10 days the parenteral nutrition was replaced with a special elemental diet (Alitraq®) and a pancreatic enzyme capsule (CREON® 2500).
Treatment for tuberculous chylothorax includes antituberculous therapy, a low-fat diet, pleural fluid drainage tube placement, and steroids, an effective treatment reported in various cases in the literature[4]. In some cases thoracic duct ligation and the use of octreotide is required[4]. In the case described above, antituberculous therapy was initiated once the etiology of chylothorax was confirmed by the presence of an acid-fast smear and was followed by clinical and radiological improvement.
This case has clinical, diagnostic, and therapeutic characteristics similar to those of other cases of chylothorax associated with tuberculosis reported in the literature. In a systematic review by Rajagopala[4], 60.6% of cases presented dyspnea; 54.5%, cough; 21.6%, left-sided pleural effusion; and 27%, isolation of M. tuberculosis in sputum or BAL samples. Treatment consisted of closed pleural drainage and a low medium-chain-fat diet for 63% of cases, and antituberculous treatment was associated with a survival rate of 94.4%[4].
Conclusions
The case of tuberculous chylothorax described above highlights the importance of evaluating tuberculous etiology when no prior trauma or thoracic surgery exists, especially in countries like Peru, where pulmonary tuberculosis is a prevalent disease. It also highlights the importance of starting antituberculous treatment in a timely manner, when there is diagnostic suspicion of the infection, for quick resolution of chylothorax.
Notes
Contributor roles
LARH: conceptualization, methodology, formal analysis, research, resources, writing - preparation of the original project, writing - review and editing, visualization, supervision, project administration. LACU: conceptualization, methodology, formal analysis, research, resources, writing - preparation of the original project, writing - review and editing, visualization, supervision, project administration. JLCP: conceptualization, methodology, formal analysis, research, resources, writing - preparation of the original project, writing - review and editing, visualization, supervision, project administration. ONAH: conceptualization, methodology, formal analysis, research, resources, writing - preparation of the original project, writing - review and editing, visualization, supervision, project administration. DAAV: conceptualization, methodology, formal analysis, research, resources, writing - preparation of the original project, writing - review and editing, visualization, supervision, project administration. MJCZ: conceptualization, methodology, formal analysis, research, resources, writing - preparation of the original project, writing - review and editing, visualization, supervision, project administration. DCRC: conceptualization, methodology, formal analysis, research, resources, writing - preparation of the original project, writing - review and editing, visualization, supervision, project administration.
Acknowledgments
The authors would like to thank Dr. José Cárdenas-García f or his careful review of the manuscript and useful suggestions. We also thank the Trujillo Center of Excellence in Tuberculosis (Centro de Excelencia en Tuberculosis “Luz Caviedes Rojas”, CENEX-Trujillo) at the Hospital Regional Docente de Trujillo for providing the space for this study as well as the database of the clinical history, the diagnostic images, and the patient laboratory tests.
Ethical aspects disclosure
The informed consent requested by Medwave has been signed by the patient and a copy of the signed form forwarded to the editorial board of the Journal.
Competing interests
The authors have completed the ICMJE conflict of interest declaration form and declare that they have not received funding for the completion of the report; have no financial relationships with organizations that might have an interest in the published article in the last three years; and have no other relationships or activities that could influence the published article. The forms can be requested from the lead author or the Journal editor.
Funding statement
The authors declare that there were no external sources of funding.

