Estudios originales
← vista completaPublicado el 17 de abril de 2024 | http://doi.org/10.5867/medwave.2024.03.2758
Control metabólico de pacientes diabéticos tipo 2 tratados con empagliflozina: serie de casos
Metabolic control in type 2 diabetic patients treated with empagliflozin: A case series
Abstract
Background Type 2 diabetes mellitus is a highly prevalent disease and is associated with increased morbidity and mortality. Due to the low percentage of adequate glycemic control, new strategies for the treatment of type 2 diabetes mellitus have been sought, including sodium-glucose cotransporter type 2 inhibitorss.
Objective To describe the evolution of patients with type 2 diabetes mellitus with insulin requirements treated with empagliflozin at the Peñaflor Hospital. The primary objective was to evaluate the efficacy of the medication regarding glycosylated hemoglobin A1c (HbA1c). The secondary objectives were: 1) achievement of HbA1c equal to or less than 7.5% according to survival analysis. 2) Change in glomerular filtration rate and urinary albumin excretion post treatment.
Methods Review of clinical records of all patients treated with empagliflozin from November 2019 to June 2023. Average follow-up of 19 (16.3 to 40) months. To compare HbA1c values according to follow-up ranges, the paired T test or Wilcoxon test was used.
Results We included 58 patients, 15 men and 43 women (74.1%), with an average age of 58.5 ± 9.2 years, ranging from 35 to 75 years. Baseline HbA1c of 10.3 ± 1.6% and 8.98% ± 2.2 in a follow-up of 18 to 24 months post-treatment, resulted in a decrease of 1.27% (p = 0.002; confidence interval 95%: 0.5 to 2.03). The most common adverse effect was urinary tract infection.
Conclusions Patients with type 2 diabetes mellitus with insulin requirements treated with empagliflozin at the Peñaflor Hospital achieved better glycemic control with few adverse effects.
Main messages
- Type 2 diabetes mellitus is a highly prevalent disease and is directly associated with cardiovascular disease, one of the leading causes of mortality worldwide.
- Sodium-glucose cotransporter type 2 inhibitors are a relatively new treatment for type 2 diabetes mellitus and have been shown to reduce cardiovascular events and improve metabolic control.
- The present study describes the improved metabolic control and low incidence of adverse effects in insulin-requiring type 2 diabetic patients treated with a sodium-glucose cotransporter type 2 inhibitor in a Chilean public hospital.
- Since this is a single-center, non-randomized, observational study with no control group, the projection of the findings of this study to the rest of the population is limited, together with the small number of the sample and the initiation of therapy at diverse points in time.
Introduction
Type 2 diabetes mellitus is a highly prevalent disease in Chile, occurring in 12.3% of people over 15 years of age, according to the National Health Survey 2016-2017 [1]. This pathology is associated with increased morbimortality and rate of years of life potentially lost. Between 2000 and 2016, there was a 5% increase worldwide in premature mortality due to diabetes [2]. In addition, it is a known risk factor for dialysis admission, coronary heart disease, stroke, and diabetic retinopathy, among others [3,4].
The achievement of therapeutic objectives represents a real global challenge, and no successful figures have been obtained. In Chile, 86% of diabetics are aware of their condition, and 58.2% are under treatment, among which 39.4% have good metabolic control with glycosylated hemoglobin A1c values below 7% [5].
Due to the low percentage of adequate glycemic control, new strategies for the treatment of type 2 diabetes mellitus have been sought, including sodium-glucose cotransporter type 2 inhibitors.
The Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes (EMPA-REG OUTCOME) study [6] evaluated the efficacy of empagliflozin in reducing cardiovascular mortality as a primary endpoint, achieving a statistically significant result. Furthermore, in this clinical trial, a decrease in glycosylated hemoglobin A1c was recorded with empagliflozin 10 and 25 milligrams, showing a decrease of 0.54 and 0.6%, respectively, evaluated at 12 weeks of follow-up compared to placebo.
After this study, several clinical trials with other sodium-glucose cotransporter type 2 inhibitors were published, which confirmed their effectiveness in metabolic control and positive cardiovascular and renal effects [7,8,9,10,11].
Both the American Diabetes Association Standards of Medical Care Guidelines and the Consensus of the Chilean Society of Diabetology for the Comprehensive Management of Patients with Type 2 diabetes mellitus of 2022 recommend the early use of sodium-glucose cotransporter type 2 inhibitors in patients with cardiovascular disease, heart failure or chronic kidney disease [12,13]. These guidelines suggest their use as a second drug (after metformin) if the individualized glycosylated hemoglobin A1c target is not achieved [13].
The present study describes the evolution of 58 insulin-requiring diabetics with glycosylated hemoglobin A1c greater than 7.9% and requiring high-dose insulin, treated with empagliflozin in a tertiary care hospital of medium complexity belonging to the Chilean public health system. This center attends to a high percentage of patients in rural areas and/or socioeconomic vulnerability.
The primary objective was to evaluate the drug’s efficacy concerning glycosylated hemoglobin A1c. The secondary objectives were to record:
-
The probability of achieving a glycosylated hemoglobin A1c lower than 7.5%, according to survival analysis.
-
The change in glomerular filtration rate and urinary albumin excretion post-treatment.
Methods
Design
Observational, descriptive, single-center, retrospective study.
Inclusion criteria
Inclusion criteria were patients with insulin-requiring type 2 diabetes mellitus with neutral protamine Hagedorn (NPH) insulin greater than or equal to 16 international units; glycosylated hemoglobin A1c greater than 7.9%; monitored at Hospital Peñaflor by an internist and referred from primary health care centers according to the protocol of the Servicio de Salud Metropolitano Occidente; under treatment during the entire study period.
Exclusion criteria
Exclusion criteria were established as having type 1 diabetes mellitus, chronic kidney disease stage IV or higher, acute renal failure, recent severe acute illness, life expectancy less than six months, glycosylated hemoglobin A1c less than 7.9%, and no record of glycosylated hemoglobin A1c prior to treatment.
Data confidentiality was maintained. Informed consent was not requested because it was an observational and retrospective study of a drug approved by the Institute of Public Health of Chile. Authorization was obtained from the Scientific Ethical Committee of the Hospital San Juan de Dios of Santiago, based on the postulates of the Declaration of Helsinki.
Baseline characteristics and intervention
The treatment of the 58 patients included in the study was basal-bolus insulin and non-pharmacological measures. Empagliflozin 10 milligrams per day orally was added, increasing to 25 milligrams when the glycosylated hemoglobin A1c goal was not achieved in 64% of them (n = 37).
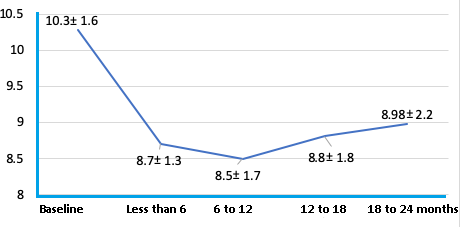
The primary objective was to determine the decrease in glycosylated hemoglobin A1c after starting treatment. This was evaluated by comparing the baseline value (prior to empagliflozin) versus the following months' ranges: less than 6, 6 to 12, 12 to 18, and 18 to 24.
The first secondary objective was to evaluate the achievement of glycosylated hemoglobin A1c equal to or less than 7.5% according to survival analysis for up to 24 months.
The second secondary objective was to evaluate the change in glomerular filtration rate and urinary albumin excretion pre- and post-treatment.
The date of the last follow-up for glycosylated hemoglobin A1c values was the last known laboratory value as of June 3, 2023.
Values for glomerular filtration rate, urinary albumin excretion, treatment-associated adverse effects, cerebrovascular disease, amputations, and deaths were recorded according to information available as of June 2022.
Records
The authors obtained the information from electronic medical records and pharmacy records.
Regarding the baseline situation, sex, age, insulin dose, metformin use, glycosylated hemoglobin A1c, presence of cardiovascular disease, arterial hypertension, glomerular filtration rate, presence of stage III chronic kidney disease, and urinary albumin excretion (in milligrams per gram of albuminuria/creatininuria) were described. The variables glycosylated hemoglobin A1c, urinary albumin excretion, and glomerular filtration rate were obtained from electronic laboratory records.
The following potential adverse effects were established: urinary tract infection, genital candidiasis, acute renal failure (defined as creatinine increase by 0.3 milligrams per deciliter), hypoglycemia, and diabetic ketoacidosis [14].
Statistical analysis
Data were processed and recorded using Microsoft Excel software, and statistical tests were performed using RStudio and Stata software.
Continuous variables were reported as mean and standard deviation or median and interquartile range. Categorical variables were recorded as numbers and percentages.
Variables were analyzed according to normality. For comparison between baseline and post-treatment, the Student’s paired-terms t-test (for glycosylated hemoglobin A1c and glomerular filtration rate) or the Wilcoxon test (for urinary excretion) was used. A value of p < 0.05 was established as statistically significant, obtaining 95% confidence intervals for the differences.
Results
In total, 64 patients started treatment with empagliflozin. Six cases were excluded from the analysis. One was because treatment was initiated in the private system, and baseline glycosylated hemoglobin A1c was unavailable. Another is due to discontinuation of the drug secondary to exanthema. Three others as a consequence of discontinuation of therapy before June 2022 due to abandonment of controls, and one due to death.
The analysis included fifty-eight patients: 15 men (25.9%) and 43 women (74.1%). Age in June 2022 was 58.5 ± 9.2 years, ranging from 35 to 75 years.
The baseline situation is described in Table 1. There was a high percentage of hypertensive patients, and median urinary albumin excretion was in the normal range. The mean baseline glycosylated hemoglobin A1c was 10.3%, ranging from 7.9% to 14.2%.
Regarding treating type 2 diabetes mellitus, most patients were on high-dose insulin before adding empagliflozin (Neutral protamine of Hagedorn insulin ranging from 16 to 130). Only two patients were on glargine, one on degludec, and one on ultrarapid.
Metabolic control and complications
The evolution after treatment with empagliflozin (primary objective) is described in Table 2. A statistically significant decrease in glycosylated hemoglobin A1c between baseline versus all follow-up ranges stands out.
In Figures 1 and 2, when analyzing by survival analysis, the achievement of a glycosylated hemoglobin A1c equal to or less than 7.5%, it is observed that:
At 12 months, 62% had no glycosylated hemoglobin A1c greater than 7.5% (95% confidence interval: 0.48 to 0.73). 38% of patients had at least one glycosylated hemoglobin A1c less than 7.5% at that follow-up.
Glycosylated hemoglobin A1c less than 7.5%.
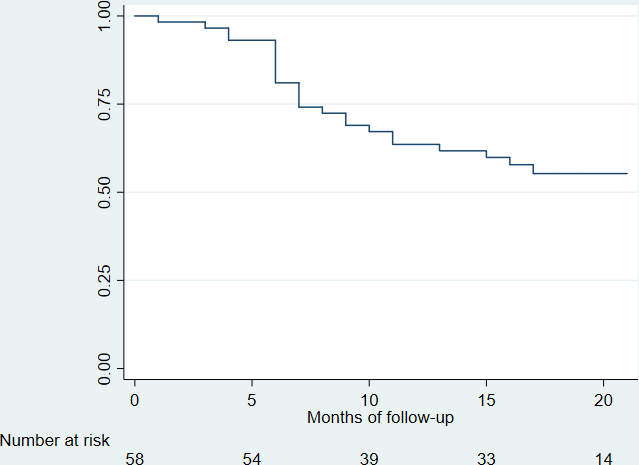
Glycosylated hemoglobin A1c according to months of follow-up.
At 24 months, 55% had no glycosylated hemoglobin A1c greater than 7.5% (95% confidence interval: 0.41 to 0.67). 45% of patients had at least one glycosylated hemoglobin A1c less than 7.5% at that follow-up.
Figure 2 shows the variation of glycosylated hemoglobin A1c in the same ranges and patients described in Table 2. A greater decrease was observed in the first six months, with a tendency to increase in the following controls, although remaining below the baseline level and with a significant decrease.
Concerning renal function, there was no statistically significant difference in urinary albumin excretion or glomerular filtration rate, as described in Table 3.
Regarding the complications possibly associated with the treatment, the most frequent were genital candidiasis (whose diagnosis was mainly based on pruritus and genital secretion) and urinary tract infection (according to urine and urine culture). There were no cases of diabetic ketoacidosis or Fournier’s gangrene during the follow-up period. In the case of hypoglycemia, the respective pharmacological or dietary adjustments were made. Table 4 specifies the rest of the complications described as probably related to empagliflozin.
The only death was interpreted as unrelated to treatment since it was a case with a long evolution of the disease and the presence of previous cardiovascular complications. The cause of death was septic shock secondary to diabetic foot.
The only case of limb amputation was secondary to peripheral arterial disease with poor response to revascularization and was therefore not related to the drug. This patient reinitiated empagliflozin after discharge, with good clinical evolution and improved glycemic control.
There was no acute myocardial infarction or stroke during the follow-up period. There were two cases of acute heart failure.
Discussion
In our registry, insulin-requiring type 2 diabetic patients with glycosylated hemoglobin A1c greater than 7.9% (mean 10.3%) treated with empagliflozin had an average decrease in glycosylated hemoglobin A1c of 1.27% after 18 to 24 months of follow-up after initiation of therapy. These results are comparable with national [15] and international experience [6]. In the study by Contreras et al. [15], the baseline glycosylated hemoglobin A1c was 8.4%, and in EMPA-REG OUTCOME [6], it was 8.1%, decreasing to 7.6 and 7.8%, respectively. Probably, the greater decrease in glycosylated hemoglobin A1c in our group was due to higher baseline values.
In the Contreras study and EMPA-REG OUTCOME, insulin use was nearly 50%. In the present study, patients had worse baseline metabolic control, and all required insulin (most of them in high-dose basal-bolus). Our patients generally did not decrease insulin doses, probably due to the greater severity, time of evolution, relative insulinopenia, and complexity of the disease.
When analyzing survival with the response variable of achieving at least a glycosylated hemoglobin A1c less than or equal to 7.5% during follow-up, we see that a significant percentage achieved this goal (38% at 12 months). However, a subsequent increase in glycosylated hemoglobin A1c during follow-up above that target is observed in several patients (mean glycosylated hemoglobin A1c of 8.98 from 18 to 24 months post therapy).
The drug was generally well tolerated, with 14% of genital candidiasis and 24% of lower urinary tract infections. As described in the literature [6], the most frequent adverse effects were mild genitourinary tract infections treated with antimicrobials and not associated with mortality. The absence of a significant difference in urinary albumin excretion is a result similar to EMPA-REG OUTCOME [6], which differs from Neal et al. [8] and Perkovic et al. [10]. These studies were performed with canagliflozin, in which there was a statistically significant decrease in urinary albumin excretion.
The absence of acute myocardial infarction or stroke during the follow-up period would support this drug’s known cardiovascular beneficial effect [16]. However, the short follow-up period and the absence of comparison versus placebo do not allow us to ensure this association.
There was an important percentage of hypoglycemia reported (20%), which could be attributed to the high doses of insulin used and the time of evolution of the disease.
Another aspect to highlight was the low incidence of acute renal failure, of low severity and which in no case required permanent suspension of the drug. Wanner et al. [7] describe that the requirement for renal replacement therapy at three years of follow-up decreased by 50%. Therefore, our group of patients may benefit from a lower requirement for dialysis in the future, resulting in a better quality of life for them and less expenditure of resources for the health care system. This, is considering not only the better metabolic control seen in this group but also the known beneficial effects at the cardiovascular, lipid, anti-inflammatory, renal, hematological, and weight levels [17].
Although our registry sought to evaluate effectiveness in terms of lowering glycosylated hemoglobin A1c, we believe it is essential to highlight the beneficial effects other than glycemia reported in multiple studies [7,8,9,10].
Limitations
The small sample size limits the external validity of the study. Being a single-center, non-randomized, observational study with no control group, the projection of the findings to the rest of the population is limited. For the same reasons, the potential cardiovascular benefit cannot be assured. Other variables in which benefit could also have been demonstrated, such as body mass index and blood pressure, were not recorded. Although lower than baseline, the mean glycosylated hemoglobin A1c at the end of follow-up was still high.
Finally, by initiating therapy at diverse points in time (between June 2019 and April 2022), follow-up times had high variability, limiting the projection of results.
Conclusions
Considering the little reported experience with type 2 sodium-glucose cotransporter inhibitors in our country, this study and Contreras et al. [15] support that this family of drugs, applied to patients with poorly controlled type 2 diabetes mellitus, is safe and effective.
In conclusion, patients with type 2 diabetes mellitus treated with empagliflozin at Peñaflor Hospital achieved better glycemic control and had few adverse effects. Considering the international evidence and our reported experience, we believe that the availability of the drug in the Chilean pharmacological arsenal could help to achieve therapeutic objectives.

