Estudios originales
← vista completaPublicado el 14 de octubre de 2024 | http://doi.org/10.5867/medwave.2024.09.2801
Diagnóstico y etapificación de cáncer de mama en Chile: estudio por encuesta no probabilística de III a IV frecuencia y tiempos
Breast cancer diagnosis and staging in Chile: A non-randomized survey-based study to assess frequency and delays
Abstract
Introduction Breast cancer progression involves physiological mechanisms such as metastasis. Delays in diagnosis and treatment increase the risk of mortality and are associated with barriers to healthcare access. In Chile, breast cancer is highly prevalent, and early diagnosis has improved, although disparities in the disease evolution persist. This study characterized diagnostic and staging tests, waiting times, and sociodemographic profiles to identify delays and inequities in care.
Methods Survey study. Using a non-probabilistic sample, a questionnaire was applied in an encrypted platform with prior informed consent. The instrument collected data on requested tests, associated times, staging, and sociodemographic characteristics. These variables were analyzed using descriptive statistics, tests of association, confidence intervals, and comparison tests using bootstrapping.
Results A sample of 263 persons was obtained. The most requested tests were biopsy (99.62%) and blood tests (80.23%). The median number of tests requested was six (Q1:4, Q3:8), with a mean of 5.87 (standard deviation: 2.24). No significant differences were observed in the percentage of persons from whom the total number of examinations were requested according to the studied variables. The day-hour-result intervals ranged from 1 to 365 days. The median day-hour-result of the biopsy was 15 days (Q1:10, Q3:30). People between 40 and 49 years old, non-residents of the capital city, belonging to income quintile I, with high school education, from the public health system, with late-stage diagnosis had higher median day-hour-result in biopsy. There was no significant difference in the number of requested tests according to staging (I and II, or III and IV).
Conclusions Biopsy in Chile is the test of choice for diagnostic confirmation in breast cancer. Other tests are requested regardless of the diagnosis stage, contrary to the recommendations of clinical guidelines. Cancer prognosis is crucial, especially in countries with greater inequalities.
Main messages
- Breast cancer is a global problem, and Chile faces challenges in its diagnosis and treatment. Diagnostic delays are related to access barriers and socioeconomic factors.
- This research is novel because it addresses the management of breast cancer examinations in Chile from the viewpoint and experience of the users of the healthcare system.
- The results showed that multiple tests were requested regardless of the cancer stage (I to II, III to IV), with biopsy being the most requested and most delayed test.
- This study’s limitations are the research design and sample size, which did not allow for establishing causal relationships.
Introduction
The progression of breast cancer includes specific physiological mechanisms such as metastatic capacity, increased angiogenesis, evasion of apoptosis, and unlimited cell divisions, among others [1], and there is a continuous search for new prognostic biomarkers for clinical management and improved outcomes of care [2]. Although the physiological contribution to the prognosis of breast cancer is indisputable, it is relevant to consider the impact of cancer in different population groups and in low- and middle-income countries with greater inequalities and vulnerable populations [3,4]. In turn, it is important to recognize that access to timely diagnosis and treatment is fundamental to improving healthcare outcomes for patients with cancer [2].
Diagnostic and treatment delays in breast cancer have been associated with an increased mortality risk [5,6,7,8,9]. These delays may be related to healthcare access barriers [10]. Such barriers mainly affect low- and middle-income countries [11] and populations living at socioeconomic disadvantage [12,13,14,15,16]. The literature does not identify a universal consensus on diagnostic and treatment intervals for better prognosis [11,17] in breast cancer. However, a reported interval to identify delays in this neoplasm consists of more than three months between detecting symptoms and initiating treatment [10,17,18,19,20].
Breast cancer is a global healthcare concern. In 2020, it was the most diagnosed cancer in the world, with 2.2 million cases, according to the World Health Organization [21]. In Latin America, this was the most frequent cancer in women [22], and in recent decades, there has been an increasing trend in mortality [23]. Chile ranks sixth in breast cancer incidence in Latin America [22]. Data obtained from the Global Cancer Observatory show that breast cancer is the second most common cancer in the country, with an age-adjusted rate of 17.7 per 100 000 inhabitants [24] and a mortality rate of 10.2 per 100 000 inhabitants [24]. According to the Department of Information and Statistics of the Ministry of Health, the adjusted mortality rate of this cancer between 1990 and 2015 was 12.9 women died per 100 000 inhabitants [25].
In Chile, when analyzing cancer mortality from the social determinants of health approach, the main disparities are demographic (sex, age, place of residence), socioeconomic (education, household income), and healthcare system (type of health insurance) [12,13,14,15,16]. In breast cancer, national studies have identified differences in mortality according to place of residence (region, urban, rural) [26] and educational level [27]. To address these differences, in 2005, breast cancer was included in the plan of Explicit Health Guarantees (GES) [28]. One of the universal guarantees of this plan in breast cancer establishes that the interval between suspicion and diagnosis should not exceed 45 days. Likewise, the interval between diagnosis and staging should not exceed 45 days. In addition, the clinical guidelines for this cancer provide recommendations related to diagnostic confirmation and staging tests [28]. For diagnostic confirmation, the recommended test is percutaneous breast biopsy. In addition, other complementary tests are not recommended in asymptomatic stage I and II patients. For staging and extension studies in patients with suspected systemic involvement, chest radiography and/or computed axial tomography of the chest, ultrasound of the abdomen-pelvis and/or computed axial tomography of the abdomen-pelvis, bone scintigraphy, and magnetic resonance imaging are recommended. The management of hours, execution, and obtaining the results of these exams represent the patient’s navigation in the system, ideally with the support of the treating team. These times are critical to achieving compliance with the Plan of Explicit Health Guarantees in breast cancer.
Data from Chile in recent years have shown an increase in breast cancer diagnoses in early stages (In Situ, I and II), a trend towards an increase in stage IIA diagnoses [29], and a reduction in stage IV [28]. However, differences persist between groups of women according to socioeconomic level, type of insurance, and region of residence [12,13,14,15,16].
Currently, in Chile, there is no detailed research on the patients' experience concerning access to diagnostic and staging for breast cancer in Chile. Nevertheless, this is an essential and indispensable process for diagnosis and treatment selection, representing a gap to be considered. Studying this problem could allow us to identify whether there are delays in the diagnostic and staging processes, as well as to explore inequities associated with specific characteristics when stratifying by clinical (staging), sociodemographic (age, sex, region of residence, educational level, household income) and health system (type of healthcare system) determinants. Considering the above, this research aimed to characterize the profile of breast cancer diagnostic and staging examinations in Chile in terms of requested exams, time intervals between requesting the exam and getting time to perform them and the delivery of results, and the sociodemographic profile of the participant.
Methods
Design
A retrospective, cross-sectional, retrospective, quantitative, retrospective survey nested in a multimethod study of therapeutic trajectories of breast cancer experience in the Chilean health system. This work was conducted between 2021 and 2022 [30]. From the research conducted in 2022 by Cabieses, Obach, Espinoza, and Rodriguez, the samples related to breast cancer for this study were extracted, as well as the databases of the variables of interest for the stated objective. The characteristics and results of this study are available in the institutional repository of the Universidad del Desarrollo with open-access [30].
Sample
The target population was adults who reported to be living or to have lived with breast cancer at the time of answering the survey. In addition, they had to have received healthcare services in public or private facilities (hospitals, private clinics, primary care centers, etc.) of the Chilean healthcare system.
A sample was drawn from the target population based on a non-probabilistic sampling design (convenience sampling allows selecting those accessible cases that agree to be included based on convenience or proximity). For this purpose, participants were recruited through contact networks generated through permanent links with healthcare services, public and private healthcare teams, and organized groups of cancer-related patients nationwide. These contact networks informed people about the profile of the study’s target population. For this purpose, an informative poster providing the contact details of the study coordinator was handed out, with whom they could directly answer the questionnaire via telephone or WhatsApp. They could also enter directly to answer the questionnaire via QR code. Additionally, to expand the sample, dissemination posters were created for social networks, open seminars, radio and press appearances, and emails inviting participation that included the link to the online survey.
Upon entering the survey on the encrypted Alchemer platform, there were rigorous questions to check the inclusion criteria for all participants. The inclusion criteria were:
-
To have lived or to be living with breast cancer.
-
To be 18 years of age or older.
-
To be treated in Chile’s public or private healthcare system.
-
Have access to the Internet (personal or through the survey coordinator) or a telephone call to answer the survey.
-
Have the cognitive abilities to understand and respond to the survey questions.
The recruitment process (between August 2021 and April 2022, both months included), according to the study’s timeframe.
Survey and data collection
The measurement instrument was a structured questionnaire designed by the research team based on a scoping review of international literature and according to the guidelines of the Chilean clinical guide. The instrument was previously piloted and included the following dimensions:
-
Demographic and socioeconomic profile and working conditions.
-
Medical history.
-
Forecast and perception of the healthcare system.
-
Therapeutic trajectory according to the flow of care.
-
Quality of care and overall satisfaction.
At the end of the nine-month recruitment period (August 2021 and April 2022, both months included), the participants' responses could be downloaded directly to the Alchemer platform in Excel format.
According to clinical guidelines [28], the questionnaire considered estrogen receptor, progesterone receptor, HER2, and Ki67 for tumor marker tests, confirmation, and staging for biopsy. To study the extension, chest X-ray and/or computed axial tomography, abdominal and pelvic ultrasound and/or computed axial tomography, bone scintigraphy, and resonance imaging were considered.
Study variables
-
Request for tests: through the question “What tests were requested to study the stage of your disease? “a series of tests were listed (biopsy, tumor marker tests according to clinical guidelines: estrogen receptor, progesterone receptor, HER2 and Ki67, computed axial tomography or chest scan, abdomen-pelvis ultrasound, abdomen-pelvis computed axial tomography, bone scintigram, magnetic resonance imaging, chest x-ray and positron emission tomography), indicating whether it was requested, not requested or does not know.
-
Days elapsed: the number of days elapsed between the request and the receipt of the results of each examination was consulted.
-
Examination perceived to have taken the longest: from the list of nine examinations, “Which test took the longest to complete?” was asked, and only one could be selected.
The following were considered as secondary variables:
-
Sex (male, female).
-
Age (continuous and categorized as from 40 years, from 40 to 49, from 50 to 69, and from 70 to 83 years, according to the Chilean breast cancer clinical guidelines [28] and the recommendations of the American Cancer Society [31].
-
Region of residence (Metropolitan region or other).
-
Monthly income (continuous and according to quintile).
-
Educational level (elementary, middle, high school, higher).
-
Health insurance (public, private system).
-
Complementary health insurance (have, don't have).
-
Breast cancer staging (stage II to II, stage III to IV, according to the screening recommendation of breast cancer clinical guidelines for patients over 15 years of age [28]).
The category “no response or unknown” was included in all cases.
The sociodemographic variables, type of tests requested, staging, and tests with the longest perceived delay correspond to qualitative variables. Meanwhile, the number of examinations and the delay between requesting them, the moment of performing them, and obtaining the results correspond to quantitative variables.
Statistical analysis
The following indicators were constructed based on the variables for requesting examinations:
-
Number of requested examinations (from 1 to 9).
-
Combination of requested exams.
-
Total number of requested exams.
These indicators, together with the rest of the variables, were analyzed descriptively using measures of frequency, central tendency, position, and dispersion according to the nature of the variable. The distribution type of continuous variables was assessed using the Kolmogorov-Smirnov normality test with Lilliefors correction. In addition, both the indicators constructed and the primary variables were described according to sociodemographic, economic, health insurance, complementary insurance, and cancer staging characteristics (secondary variables). All estimated percentages were accompanied by their respective confidence intervals. Regarding the sampling, confidence intervals were constructed using Bootstrap for a proportion [32]. Based on the resampling method (using 100 000 replicates), the success proportions were calculated, and the confidence interval was constructed using the corresponding Bootstrap distribution’s percentiles to determine the confidence interval’s upper and lower bounds.
Differences between the proportion of respondents who were asked to take the different types of exams (alone and stratified by secondary variables) were examined using bootstrapping proportion comparison tests. Similarly, comparisons between the time intervals between having time to perform an examination and the delivery of its result, according to specific examination and secondary variables, were performed using a bootstrapping median comparison test, applying the percentile method for differences [32]. The association between secondary and primary qualitative variables was analyzed using Chi-square or Fisher’s exact test, as appropriate. All analyses were performed using R (version 4.3.1) and Microsoft Excel software, considering 95% confidence (2.5 and 97.5 percentiles, Bootstrap) and a significance of 0.05.
Ethics
The study was approved by the ethical-scientific committee of the Faculty of Medicine, Universidad del Desarrollo, Clínica Alemana, Chile (Memorandum number 2021-67, July 26, 2021).
Results
A sample size of 263 people was achieved, with men and women between 29 and 83 years old at the time of the survey.
Sociodemographic characteristics and healthcare
Of the 263 respondents, 260 reported being female (98.86%), with only one male respondent (0.38%; two did not respond). The sample showed a higher proportion between 50 and 69 years (49.05%), with 13.31% (n = 35) of respondents in the under-40 age bracket, similar to cancer prevalence data in Chile [33]. Regarding residence, 49.81% (n = 131) resided in the capital city (Metropolitan Region), similar to the national distribution [34]. In agreement with national data [35], higher education was the most frequent at 55.13%, followed by secondary education (37.64%). On the other hand, income had a 5% trimmed mean of $1 125 098 CLP, with quintiles at the $400 000 CLP, $600 000 CLP, $1 000 000 CLP, and $2 000 000 CLP thresholds, respectively. Finally, similar to country statistics [35], 71.1% (n = 187) belonged to the public healthcare system, and 31.94% (n = 84) reported having complementary health insurance (Table 1). The percentage of participants with complementary health insurance from the private system was higher than those from the public system (61.84% versus 19.79%, p < 0.05).
Requested exams
Among the nine requested tests, the most frequent were biopsy and tumor marker tests, with 99.62% (n = 262; 95% CI: 98.86% to 100%) and 80.23% (n = 211; 95% CI: 75.29% to 84.79%), respectively. In contrast, the least requested tests were positron emission tomography, abdomen-pelvis ultrasound, and chest radiography (24% for the former and approximately 55% for the latter two), except for positron emission tomography (23.95% of requests). Imaging tests were requested in more than 50% of respondents, with chest computed tomography (78.71%; 95% CI: 73.76% to 83.65%) and bone scintigraphy (72.24%; 95% CI: 66.54% to 77.57%) being the most frequent.
Biopsy requests were significantly more frequent (p < 0.05) than the other examinations, and the same was observed for positron emission tomography. Likewise, blood tests and chest computed tomography differed significantly from abdominal ultrasound, abdomen-pelvis computed tomography, magnetic resonance imaging, and chest radiography. In contrast, abdominal ultrasound differed from scintigram and magnetic resonance scintigram (p < 0.05) (Table 2). For the total number of respondents, the median number of examinations requested was six examinations (Q1: 4, Q3: 8), with a mean of 5.87 examinations (SD: 2.24) (Table 3). 12.17% of respondents (95% CI: 8.37% to 16.35%) requested all examinations, and 3.8% (n = 10; 95% CI: 1.52% to 6.08%) only had one examination (biopsy) (Table 2).
When stratified by sociodemographic characteristics, access to healthcare, and cancer stage, no statistically significant differences were observed in the percentage of patients requested to undergo all the tests by specific groups. In descriptive terms, the difference by diagnostic stage stands out, with those in the late stage of the disease (III to IV) being the most frequently requested to have all the tests (18.2% versus 9.6%) (Figure 1).
Percentage of respondents who were asked for all tests.
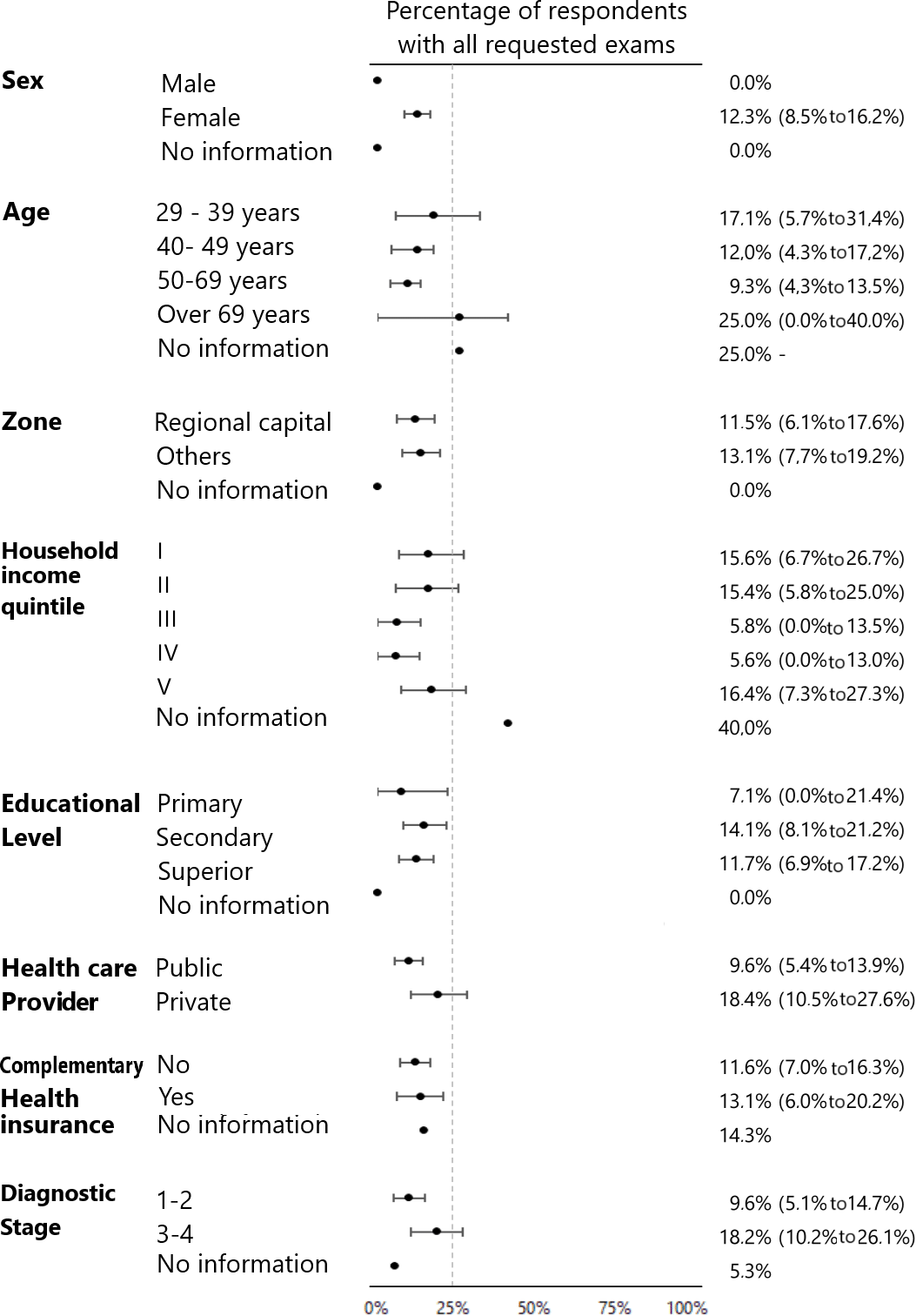
Source: Prepared by the authors based on the results of the study.
According to the report, the most frequent combination of requested tests was biopsy plus blood tests plus imaging (specifically, chest X-ray, chest computed axial tomography, abdominal ultrasound, abdomen-pelvis computed axial tomography, bone scintigraphy, and magnetic resonance imaging) (Table 3), which was specifically requested by 12.17% (n = 32) of the participants.
Days between exam performance and results
The days between performance/result ranged from one day to one year (365 days) for the various examinations. Except for bone scintigraphy and biopsy, the median days to results were seven days for the other examinations, with interquartile ranges between three and 20 days. Specifically, the median days to result for the bone scintigram was 9.5 days (Q1: 5.5 to Q3: 20) and 15 days (Q1: 10 to Q3: 30) for the biopsy. The median number of elapsed days differed significantly between biopsy and other examinations (Table 2). When stratifying by secondary variables, the median number of days between performance/result ranged from 17 to 27.5 days according to specific groups: people between 40 and 49 years of age, residents of other regions, quintile I of monthly income, middle education, public healthcare system, without complementary health insurance and in late-stage of the disease presented the highest median number of days between performance/result for biopsy. In no case did the 75% percentile of the sample exceed 45 days of waiting time (Figure 2).
Days between biopsy order and delivery of results according to sociodemographic characteristics and stage of diagnosis [29].
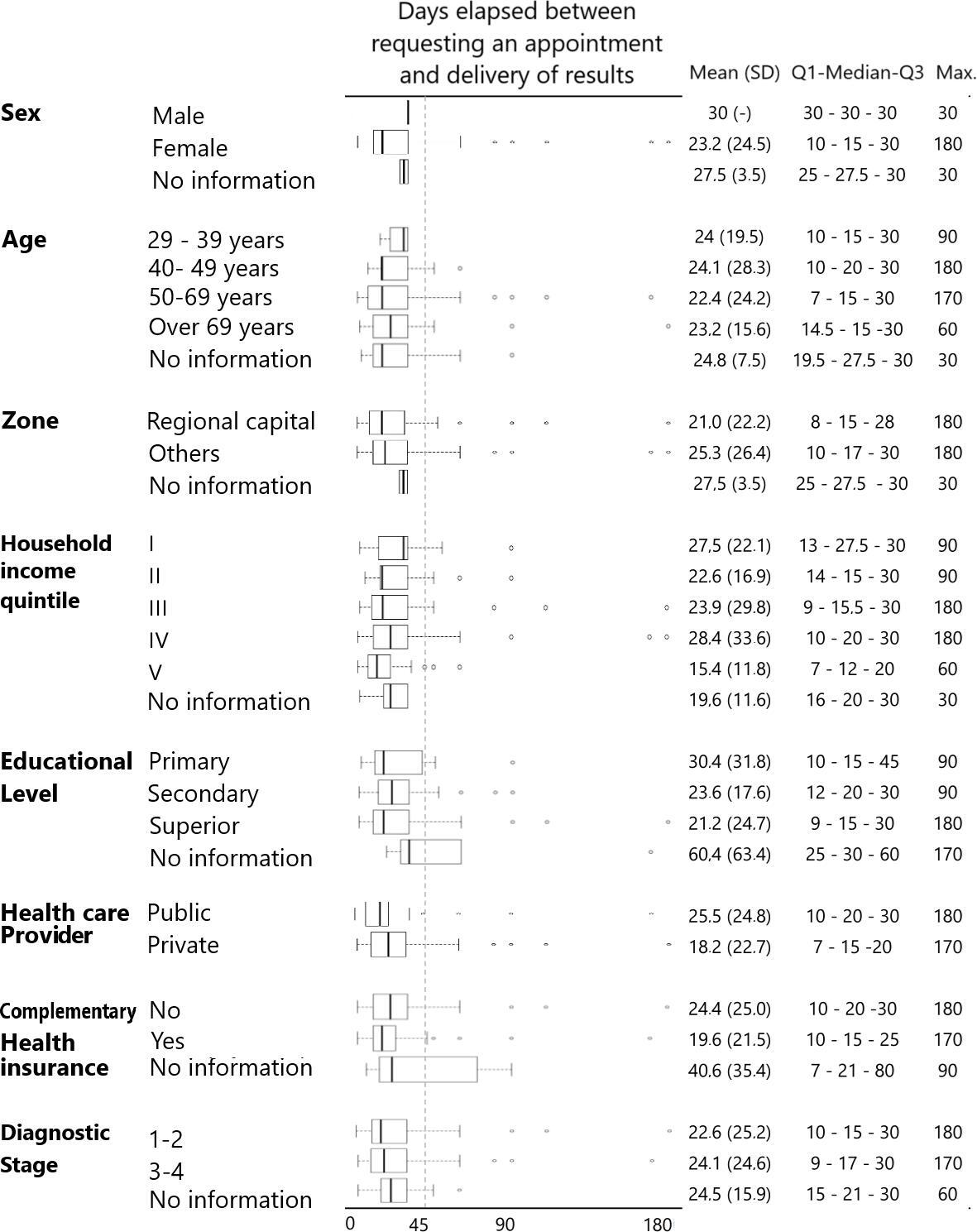
CT: computed axial tomography.
Source: Prepared by the authors based on the study results.
The perceived tests with the longest delay were biopsy (26.62%, 95% CI: 21.29 to 31.94), bone scintigram (21.29%, 95% CI: 16.35 to 26.24), and magnetic resonance imaging (10.65%, 95% CI: 7.22 to 14.45) (Table 2). After stratifying by sociodemographic characteristics, health insurance, complementary health insurance, and staging, biopsy, and scintigraphy remained in the first place (Table 3).
Disease staging
The most frequently requested tests were analyzed according to the stage of diagnosis. People with stage I and II diagnoses reported a median of 6 examinations. On the other hand, the most frequent combination for these stages was eight tests, specifically: biopsy, chest X-ray, chest CT scan, ultrasound, bone scintigraphy, abdomen-pelvis CT scan, MRI, and blood tests. A median of seven tests were requested from people in stages III and IV, with the most frequent combination being the tests mentioned earlier. In addition, it is noteworthy that in both cases, the examinations were requested from more than 50% of the people in each stage. For both cases, the test perceived with the longest delay between performance/result was biopsy (Table 3), being also the most requested test in stages I to II (100%) and III to IV (98.9%; 95% CI: 96.6 to 100). In more than 80% of the patients diagnosed in stages III and IV, the request for computed axial tomography of the thorax and bone scintigraphy stands out, a situation that differs for stages I and II where we note that, together with the biopsy, blood tests are the only ones performed in more than 80% of those surveyed (Table 4).
Some differences were descriptively observed between the percentage of respondents who claimed to be in stage III or IV at the time of breast cancer diagnosis by sociodemographic variables. For example, 34.35% of the respondents in the Metropolitan Region claimed to be at this stage, while this percentage corresponded to 3.31% for the other regions (Table 3).
Discussion
This research characterized the profile of requested tests for the diagnosis and staging of breast cancer in Chile. It analyzed the time intervals between days of performance/result for each requested test, the latter to identify possible delays. With this in consideration, differences were found in these indicators of healthcare performance and compliance with breast cancer guarantees in the plan of Explicit Health Guarantees defined by law in Chile, according to clinical variables and social determinants of health.
The results of the investigation revealed that the most frequently performed examination was a biopsy in 99.62% (n = 262; 95% CI: 98.86 to 100) of the participants. This result shows that biopsy in Chile is the test of choice for diagnostic confirmation under international [36] and national recommendations [28]. An interesting finding was the request for bone scintigraphy in 72.24% of the participants. This is given that, although bone metastasis in breast cancer is the most frequent [37], this test is not recommended for the diagnostic confirmation of asymptomatic stage I and II patients [28]. The number of requested examinations was also noted, with a median of six (Q1: 4, Q3: 8) and a mean of 5.87 examinations (SD 2.24).
These results show differences between the criteria established in the clinical guidelines [28] and the request for examinations mentioned by the participants. This is because the imaging tests reported by the participants are mainly recommended in the clinical guidelines for stage III and IV patients. In addition, only 31% (n = 64) of the participants stated having a diagnosis in these stages. On the other hand, no significant differences were observed in the request for examinations by social determinants of health. However, when analyzing days between performance/result, a greater delay for biopsy was observed in persons between 40 and 49 years of age, residents of regions outside the Metropolitan region, belonging to the poorest income quintile of the sample, having secondary education, attending the public healthcare system, without complementary health insurance, and being at a late stage of the disease. Considering the differences in mortality results present in the country between groups according to place of residence [26] and educational level [27], the request for tests above the recommendations and the longer interval of days between biopsy performance/results could have an impact on delays in diagnosis and initiation of treatment [5,6,7,8,9].
This research has both strengths and limitations. As a strength, this unprecedented and exploratory study in Chile recognizes the experience and voice of breast cancer patients as a primary source of information. It is also the first study in Chile and Latin America that analyzes examinations as a fundamental part of the diagnostic and staging processes. This knowledge is useful for improving diagnosis, staging, treatment times, and system performance [3,4]. This novel study in the country, involving qualitative and quantitative variables, recognizes that it has limitations associated with the possibility of making inferences about the population within the quantitative nature of the study. Specifically, the non-probabilistic sample generated may introduce biases and limitations to the validity of the hypothesis tests, as well as not being representative of the adult population living with breast cancer in Chile. Given the breadth of the target population, confidentiality, and how participants were invited, it was not possible to have a list of people from which a selection of participants could be made. Consequently, it was not possible to construct a sampling frame. To compensate for this shortcoming, an attempt was made to describe in detail the participant recruitment process. With this in mind, the study design allows the generation of hypotheses for future research.
Also, confidence intervals and comparisons were performed with resampling methods [32], which may provide a more suitable approach for inference in non-probability samples. This technique helps to estimate confidence intervals and empirical distributions of statistics without requiring assumptions about the underlying distribution of the data. On the other hand, it is recognized that the design of the study, the cross-sectional nature of the data obtained through a survey that collects the experience and perception of the respondents, does not allow for establishing causal relationships. In addition, there is a risk of bias in the self-administered questionnaire, which does not have validity and reliability analyses. Finally, it is recognized as a limitation of the study that the pandemic period may have influenced the information obtained from some of the participants, increasing the time of access to examinations and influencing the non-consultation even in the face of the need.
Likewise, future research could address the problem through medical records to avoid self-reporting biases and exclusion of certain population groups (such as those without the possibility of responding to a survey). Other alternatives are carrying out a study on a larger scale and budget that allows using probability samples or sectioning the research to address this issue in specific population groups. These specific groups can be detailed with more specific characteristics of health conditions or sociocultural characteristics of one or more countries. These studies can be extended to other Latin American countries, where increases in breast cancer prevalence have been observed.
Conclusions
This research seeks to provide new knowledge on breast cancer, focusing on the delays in diagnosis both locally and internationally, with special regard to diagnostic tests and staging necessary to initiate timely treatment. By analyzing our results in detail and considering the identified limitations and context, we aim to offer a clearer vision of this problem.
Addressing the delay in the diagnosis of breast cancer is an ethical and legal responsibility. It highlights preventable and modifiable differences in access to examinations that are fundamental for the effective and equitable management of this pathology in Chile.
In addition, this research will contribute to a better understanding of the inequities in access to breast cancer diagnostic and staging tests in Chile, according to various determinants. This is a key step to reduce diagnostic time and ensure more timely treatments [38].

