Estudios originales
← vista completaPublicado el 24 de septiembre de 2024 | http://doi.org/10.5867/medwave.2024.08.2923
Fulvestrant en la práctica clínica: análisis de efectividad en pacientes uruguayas con cáncer de mama HR+/HER2-
Fulvestrant in clinical practice: Effectiveness analysis in Uruguayan patients with HR+/HER2- breast cancer
Abstract
Introduction Fulvestrant demonstrated benefits in overall survival and progression-free survival in patients with advanced breast cancer, who are hormone receptor-positive and human epidermal growth factor receptor 2 negative. The characteristics, evolution, and survival of patients with hormone receptor-positive, HER2-negative breast cancer treated with fulvestrant were evaluated according to the national treatment coverage protocols of the National Resources Fund, with the aim of understanding the efficacy of fulvestrant in patients treated in usual clinical practice and comparing our results with those from pivotal studies.
Methods A database from the National Resources Fund covering the period from 2009 to 2022 was used. Survival curves were assessed using the Kaplan-Meier method, and differences were analyzed using the Log-Rank test.
Results A total of 1085 patients with an average age of 63,66 years were included. Following a follow-up of 14 months, the median overall survival was 16 months, and the median progression-free survival was 6 months. The presence of liver and bone metastases was associated with a shorter overall survival. Patients from the public sector and those with a better performance status experienced longer overall survival.
Conclusions Our findings provide a valuable perspective for treatment management in a context of limited resources. Overall survival and progression-free survival were somewhat lower than those reported in pivotal clinical trials. The presence of liver and bone metastases was associated with worse prognosis and survival; additionally, patients with worse performance status had shorter overall survival. These findings underscore the need for personalized therapies, opening new lines of future research.
Main messages
- Breast cancer is the most common cancer worldwide, and in Uruguay, it is the leading cause of cancer death in women.
- Fulvestrant is effective in the treatment of advanced breast cancer in postmenopausal patients who have not previously responded to anti-estrogen therapy. Available data on its use in routine clinical practice in several countries report similar results, albeit with significant variations.
- The study underlines the importance of tailoring breast cancer research and treatment to the realities of resource-limited countries. The results underline the need for personalized treatment strategies, given the observed variability in response to treatment.
- This study was conducted under conditions specific to the Uruguayan health system and did not include a control group, so the findings should be interpreted cautiously. These contextual and methodological factors may limit the extrapolation of the results.
Introduction
Breast cancer has the highest incidence of any cancer worldwide, with 2.2 million new cases in 2020 [1]. In Uruguay, cancer is one of the leading causes of death in the population, and breast cancer is the leading cause of cancer death in women [2].
Fulvestrant is a pure estrogen receptor antagonist administered intramuscularly. It has a similar affinity to estradiol and generates receptor down-regulation, thereby reducing the concentration of estrogen and progesterone receptors in a dose-dependent manner [3]. Research points out that fulvestrant is effective in the management of advanced breast cancer in postmenopausal patients who have previously failed to respond to anti-estrogen therapy [4]. Further research indicates that the efficacy of fulvestrant is proportional to the dose administered. In particular, the phase III CONFIRM study revealed that a 500-milligram dose regimen of fulvestrant is superior to a 250-milligram dose regimen, resulting in an overall survival prolongation of 4.1 months, favoring the higher dose [5].
Since clinical trials are conducted with highly selected patients under optimal conditions, the survival and safety results may not be reproducible in routine clinical practice. In real-world practice, most patients are older, have more comorbidities, and are often in less favorable conditions. Consequently, it is questionable whether the results obtained from clinical studies will be reproducible in everyday clinical practice.
Available data from the use of fulvestrant in routine clinical practice in Canada [6], China, and Turkey [7,8] suggest that the results in everyday clinical practice could be similar to those in randomized clinical trials. However, there are significant variations in the results. For example, a study in Turkey showed that median progression-free survival was nine months and overall survival was 28 months, with differences according to line of treatment and patient characteristics such as body mass index and presence of brain metastases [7]. Another Turkish study reported that using fulvestrant before chemotherapy improved survival, with a progression-free survival of 6.05 months and an overall survival of 29.70 months, highlighting the importance of optimal treatment sequencing [8]. In contrast, the reported progression-free survival in China was 14.1 months for the first-line, 11.2 months for the second-line, and 6.7 months for the third-line fulvestrant [9]. These differences highlight the need for local studies to understand fulvestrant’s efficacy in different clinical and population settings.
In Uruguay, the only data available on the efficacy of fulvestrant in women treated in routine clinical practice are those obtained from a report by the National Resources Fund in 2022 [10].
Incorporating high-cost medicines into a universal coverage system requires defined strategies for monitoring indications and evaluating outcomes. Fulvestrant, an expensive medicine, has been financially covered by the National Resources Fund in Uruguay since 2009. The Fund designed a coverage policy based on evidence review and international recommendations to achieve results similar to those of clinical trials. This policy is reviewed annually to adapt to scientific developments and the Uruguayan health context, allowing for informed and sustainable decisions. This ensures the quality, equity, and sustainability of the system.
In low- and middle-income countries, adequate financial resource management is crucial. This study intends to provide valuable data for clinicians, patients, and policymakers on the characteristics and outcomes of patients treated with fulvestrant following the National Resources Fund regulations.
This study aimed to determine disease-free survival and overall survival in patients with hr+/her2- breast cancer treated with fulvestrant in second-line or beyond.
Methods
Study design
This is an analytical longitudinal study based on secondary data.
Population and sample
The sample consisted of 1085 Uruguayan patients diagnosed with loco-regionally advanced or distant disseminated, hormone receptor-positive, and human epidermal growth factor receptor two negative breast cancer. These patients were treated with fulvestrant through the National Resources Fund from 1 January 2009 to 30 December 2022. Patients were selected by convenience.
Selection criteria
Uruguayan patients diagnosed with hr+/her2- advanced breast cancer, treated with fulvestrant in the period above.
Data was subtracted by National Resource Fund forms completed by treating physicians, including age, sex, stage at diagnosis, menopausal status, origin, health care institution, smoking, alcoholism, functional status according to the East Cooperative Oncology Group (ECOG) scale, date of treatment initiation, site of metastasis and date of death or last control.
Data collection
The following data were collected from the forms: age at diagnosis, sex, stage at diagnosis, menopausal status, origin, collective medical care institution where the patient was treated, smoking, alcoholism, functional status according to the ECOG scale (where zero indicates healthy and four indicates disabled), date treatment was started, site of metastasis and date of death or last control for the calculation of overall survival. The patients included were treated in private and public health care centers in Montevideo and other areas of Uruguay. All healthcare centers in the country request treatment with fulvestrant from the National Resources Fund. These data allow us to comprehensively characterize patients diagnosed with advanced breast cancer and evaluate their evolution under treatment with fulvestrant.
Variables
-
Exposure: treatment with fulvestrant.
-
Response (outcome): overall survival and progression-free survival.
-
Covariates: age, stage at diagnosis, menopausal status, origin, health care institution, smoking, alcoholism, ECOG functional status, site of metastasis.
Outcomes to be assessed
-
Overall survival: time from start of fulvestrant treatment to date of death from any cause.
-
Progression-free survival: time from the start of fulvestrant treatment to the disease progression or death date, whichever occurs first.
Statistical analysis
The Kaplan-Meier method was used to measure survival curves, assessing differences with the Log-Rank test. All results were considered statistically significant at p-values < 0.05 (two-tailed test).
Ethics
The ethics committee of the Hospital de Clínicas Dr. Manuel Quintela of Montevideo approved this work. Patients who received fulvestrant through the National Resources Fund from 2009 to 2022 who agreed to have their data used in health outcome evaluations were included. Patients signed a 'Consent for Use of Personal Data for Health Outcomes Assessments'. This consent allows their information to be used anonymously in assessments conducted by National Resources Fund technicians and academic or scientific bodies endorsed by the National Resources Fund, ensuring that personal data is kept confidential and that assessments are conducted to the highest standards of quality and safety. Patients could choose not to consent to the use of their data. In that case, they were not included in the database used for this study.
Results
A total of 1085 patients were included, of whom 98.8% (1072) were women and 1.2% (13) were men, with a mean age of 63.66 years. Of the patients, 51.2% (556) were from Montevideo. At baseline, 95% (1031) were Stage IV. Most tumors were estrogen receptor-positive (83.2%, 903) and progesterone receptor-positive (70.4%, 764). Regarding the presence of metastases, 78.3% (850) had bone metastases, 23.7% (257) had lung metastases, and 17.5% (190) had liver metastases. Of all patients, 41.9% (455) had exclusively bone metastases. In terms of lifestyle habits, 4.9% of patients (53) were smokers, and 0.2% (2) were alcohol consumers. Further details are presented in Table 1.
With a median follow-up of 14 months (0 to 163 months), the median overall survival was 16 months (95% confidence interval: 15 to 18 months). Overall survival at two years was 36.0%, decreasing to 7% at five and 1.2% at ten years. Median progression-free survival was six months, with a two-year progression-free survival of 13.1%, decreasing to 4.3% at five years and 1.3% at ten years (Table 2).
The presence of liver and bone metastases had a significant impact on both overall survival and progression-free survival. Patients with liver metastases showed a median overall survival of 11 months (95% confidence interval: 17 to 20), markedly shorter compared to a median of 18 months (95% confidence interval: 9 to12) in patients without these metastases (p < 0.0001) (Figure 1). Similarly, progression-free survival was significantly shorter in patients with liver metastases (p < 0.001).
Overall survival according to the presence or absence of liver metastases.
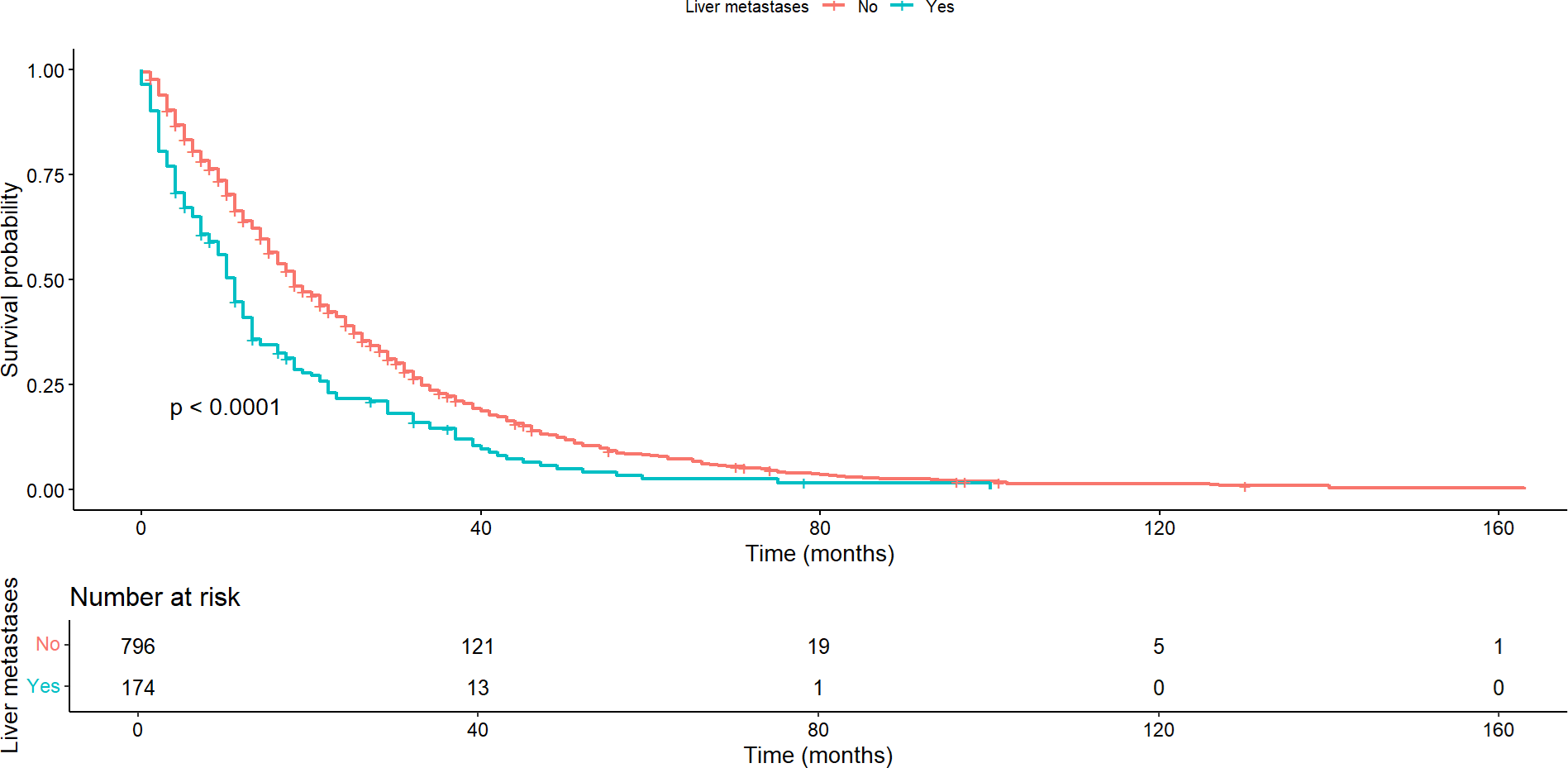
Regarding patients with bone metastases, the median overall survival was 16 months (95% confidence interval: 15 to 18) compared to a median of 18 months (95% confidence interval: 15 to 23) for those without bone metastases, with this difference being statistically significant (p = 0.0061) (Figure 2). This pattern was maintained regarding progression-free survival, where statistically significant differences were found between the groups with and without bone metastases (p < 0.001).
Overall survival according to the presence or absence of bone metastases.
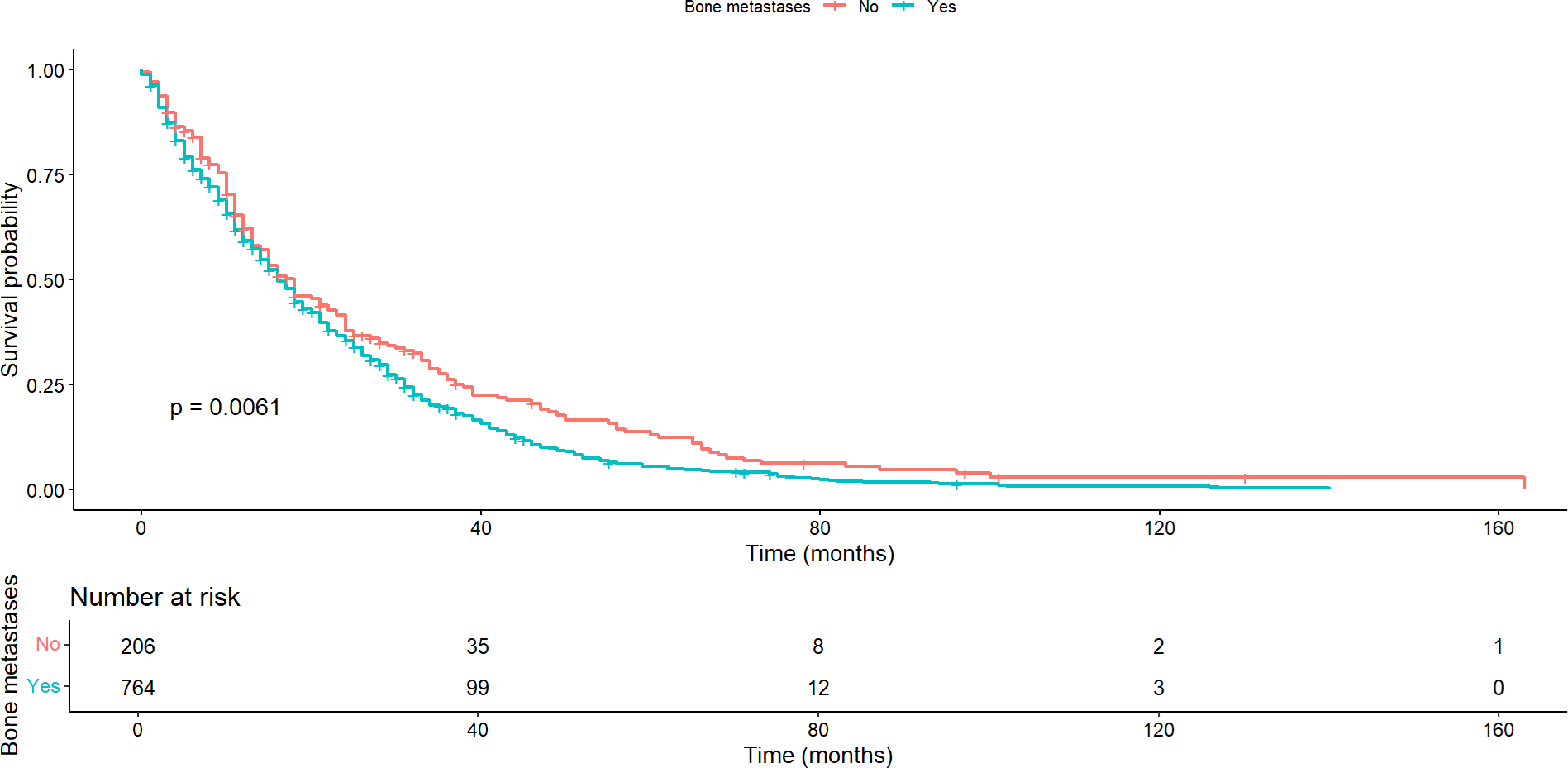
In contrast, no significant difference was observed in median overall survival between patients with and without lung metastases (p = 0.322). This pattern held true regarding progression-free survival (p = 0.939). Similarly, there was no significant difference in overall or progression-free survival between patients with and without brain metastases. In these cases, overall survival was 12 months (95% confidence interval: 7 to 37) and 17 months (95% confidence interval: 15 to 18), respectively (p = 0.446), and progression-free survival was 5 and 6 months, respectively (p = 0.421). There was no significant difference in overall and progression-free survival between patients with and without cutaneous/subcutaneous metastases. Here, overall survival was 17 months (95% confidence interval: 15 to 18) and 16 months (95% confidence interval: 12 to 21), respectively (p = 0.080), and progression-free survival was six months for both groups. Significant differences were found in overall survival by healthcare providers (p = 0.018). Survival was longer in patients treated by public health compared to those treated in private institutions (Figure 3). In contrast, there was no statistically significant difference (p = 0.271) between providers in progression-free survival.
Overall survival by health care provider.
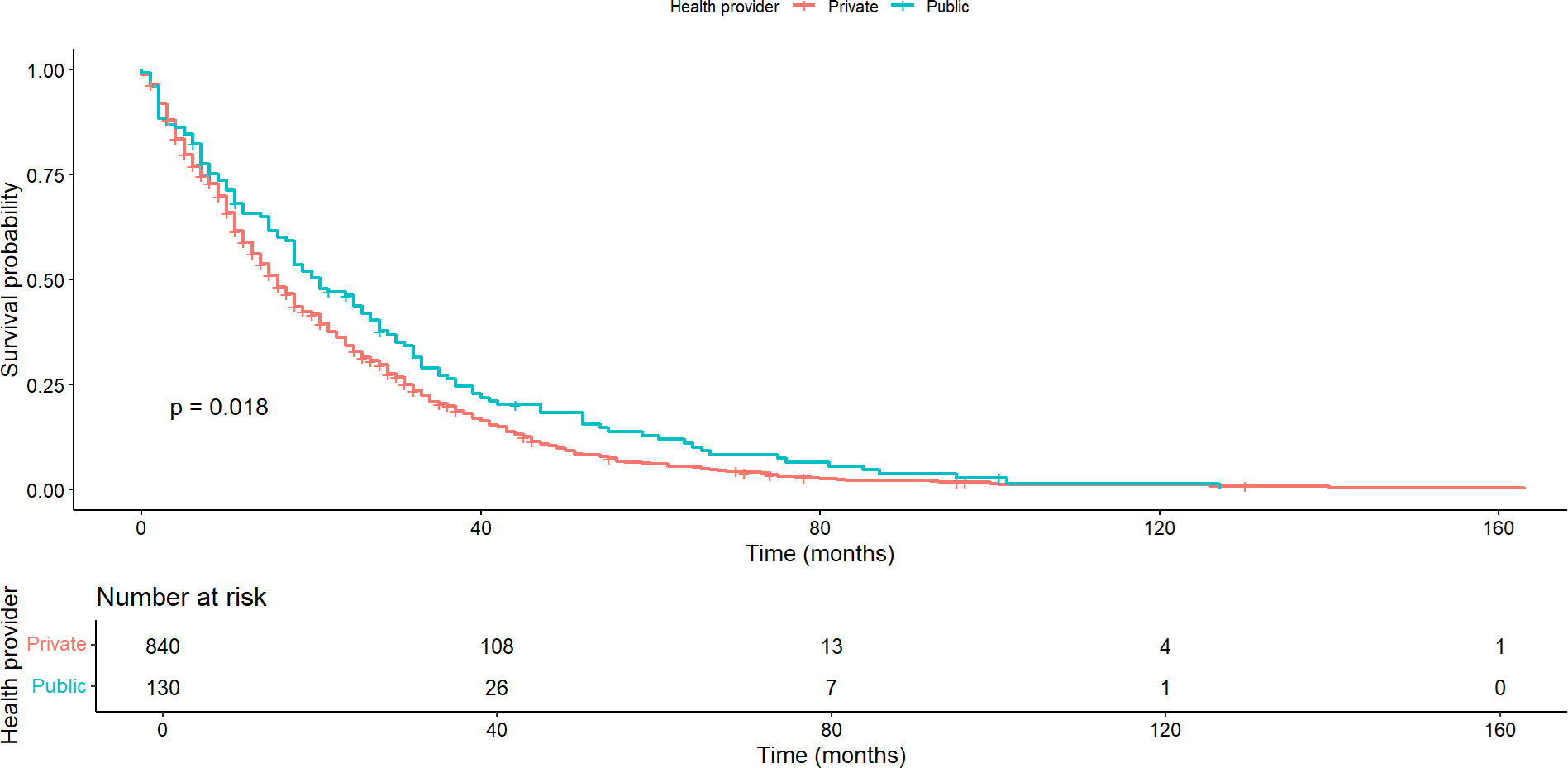
Assessment of the impact of the performance status scale on patient survival revealed significant differences in both overall survival and progression-free survival. Median overall survival was 19 months (95% confidence interval: 18 to 21), 11 months (95% confidence interval: 9 to 14), and 8.5 months (95% confidence interval: 4 to 23) for patients with ECOG performance status 0, 1 and 2, respectively (p <0.0001; Figure 4). In contrast, the median progression-free survival was seven months (95% confidence interval: 6 to 8), five months (95%: 4 to 6), and five months (95%: 3 to 15) for patients with performance status 0, 1 and 2, respectively (p < 0.00035).
Overall survival according to the functional status scale.
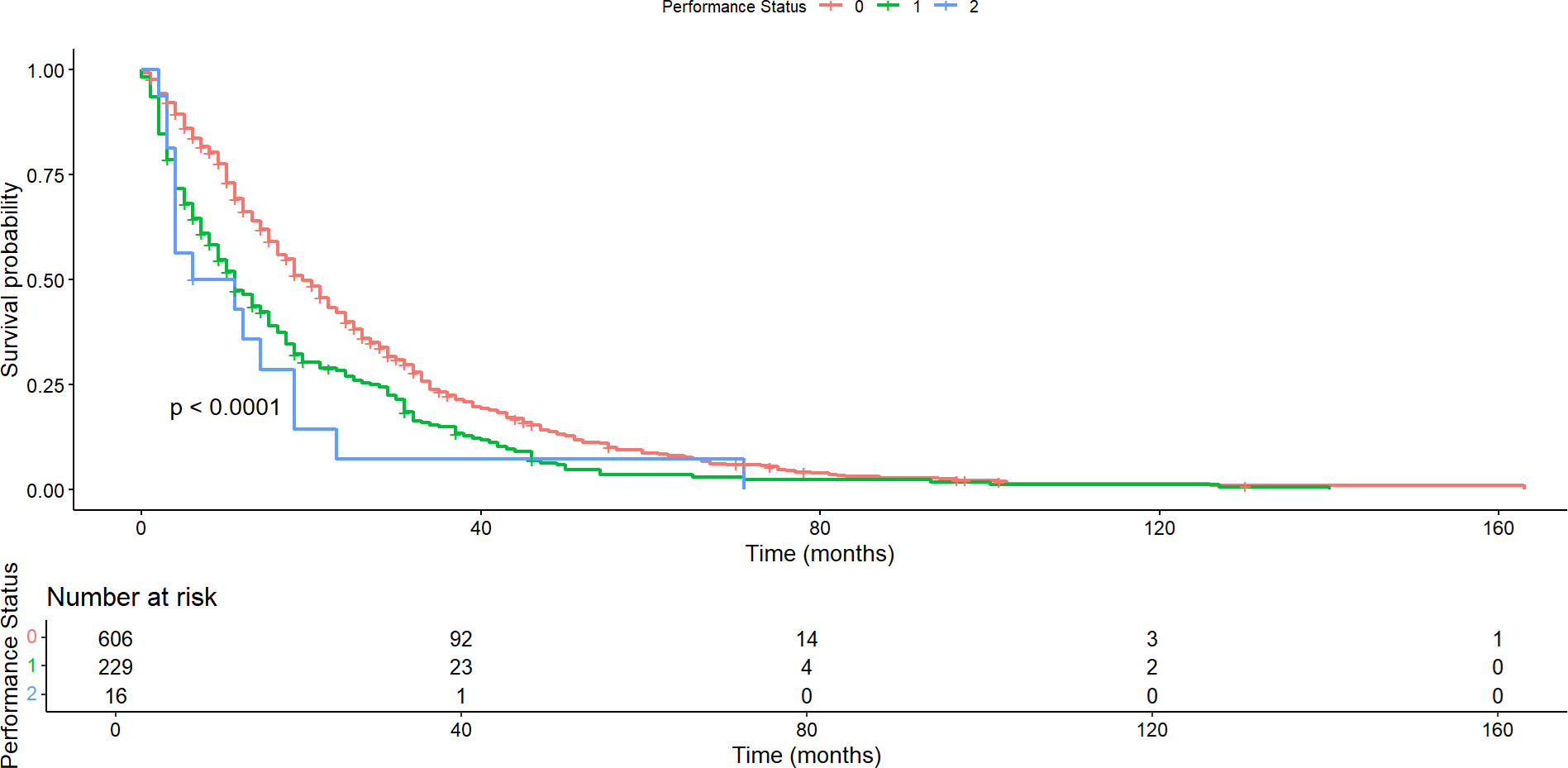
No differences were observed in overall survival (p = 0.726) nor progression-free survival (p = 0.398) between patients from Montevideo and the rest of the country.
In the Cox model for overall survival, the variables performance status scale and presence of liver metastases were significant in the simple models. These variables did not meet the proportional hazards in the multiple models, so they were used as stratification variables. The variable presence of liver metastases was also significant in the simple model. However, it did not show proportionality of risk, so for the multiple model it was incorporated as a stratification variable (Table 3).
Discussion
The allocation of resources for hormonal treatments in breast cancer represents a significant challenge, both for health policymakers and for the entities that fund these drugs [11,12]. The selective nature and ideal conditions of clinical trials often do not reflect the complexity of everyday clinical practice, where patients are generally older and have more comorbidities [13,14,15,16]. This raises questions about the applicability of clinical trial results in real-life healthcare settings.
This study provides valuable insight into the prognosis of Uruguayan breast cancer patients, revealing possible differences from clinical trial findings. The inclusion of patients in an everyday clinical practice setting provides a more representative perspective than is often seen in controlled research. In addition, findings on the efficacy of fulvestrant in everyday practice contribute to a more robust evidence base, which is crucial for making policy decisions on funding this treatment [17].
The median overall survival in the present study was 16 months, and progression-free survival was six months. These results are lower than those of the pivotal CONFIRM study [5], which reported median overall survival and progression-free survival of 25.1 and 22.8 months, respectively. They are also lower than those observed in Western patients from studies conducted in everyday clinical practice, where overall survival with second-line fulvestrant was 16 months and progression-free survival was 12 months [6], as well as in studies of Eastern patients, where progression-free survival was 11.6 months [9]. However, in the present study, progression-free survival was similar to that reported in other studies, while overall survival was lower [7,8]. When interpreting these results, it is crucial to consider that the volume of patients with brain metastases is unknown, a factor that could alter the prognosis. In addition, the treatments that patients receive after disease progression are also unknown, which could significantly affect overall survival.
Patients with a higher performance status scale had worse overall survival, with this difference being statistically significant. This is because performance status is an independent prognostic indicator, reflecting overall health status and ability to tolerate treatment. Patients with a performance status scale of 0 have a better health status and tend to have a longer overall survival than those with a higher performance status scale [18]. Regarding overall survival, the presence of cutaneous/subcutaneous or pulmonary metastases did not impact overall survival. On the other hand, the presence of liver and bone metastases was associated with shorter overall survival. This could indicate that they are associated with a worse prognosis, consistent with previous reports [19]. It is important to note that patients with extensive liver metastases were excluded in the CONFIRM study [5]. For the present study, information on liver burden was not available. This might suggest that hormone therapy alone may not be the best option in the presence of significant liver and/or bone metastatic burden. This is especially relevant, considering that combination therapy with cyclin-dependent kinase 4/6 inhibitors has been shown to improve the prognosis of patients with hormone receptor-positive advanced breast cancer [20].
This study included 1085 hormone receptor-positive and human epidermal growth factor receptor 2-negative breast cancer patients, of whom the majority were women (98.8%), with a median age of 63.66 years. This is consistent with what was reported in studies evaluating the efficacy of fulvestrant treatment in the Western population [6].
The prevalence of smoking found in the study was 4.9%, a considerably lower value compared to rates reported in the general Uruguayan population. According to data from the Global Adult Smoking Survey conducted in Uruguay during the period 2016 to 2017, the smoking rate in women was 18%, while in 2009, it was 24.1% [21]. This discrepancy suggests that there could be under-reporting of smoking data in the sample. Underreporting could be related to several factors, such as patients' reluctance to report smoking habits or lack of complete documentation in medical records. Future research should address these potential biases and seek methods to obtain more accurate data on smoking among breast cancer patients in Uruguay.
Among the strengths of the present study, the sample size is noteworthy. A substantial number of patients were included, which provides a robust database for analysis and increases the reliability of the results, as well as a long follow-up period. In addition, patients from everyday clinical practice were included, a crucial strength as it reflects a wider variety of clinical situations than controlled trials. This allows for better generalisability of the results to the general population, providing valuable information on the effectiveness of fulvestrant in a practical setting and in patients with different comorbidities. These findings are especially relevant for decision-making in medical practice and public health policy.
Limitations of the present study include the reliance on the secondary database provided by the National Resources Fund, restricting access only to data recorded and shared by them. Moreover, reporting bias may present due to a lack of details on why patients stopped receiving fulvestrant. Although disease progression appears to be the leading cause, there is a potential bias for those who were lost to follow-up or discontinued treatment for other reasons. In addition, the absence of data on post-fulvestrant therapies limits the analysis of overall treatment efficacy. This factor is relevant, as subsequent therapies may significantly influence patients' overall survival, which could explain, at least in part, the results obtained for overall survival but not for progression-free survival. In this context, we also lacked data on central nervous system metastases, which is relevant to understanding disease progression completely.
Conclusions
To our knowledge, this study represents the first comprehensive analysis in Latin America of patients diagnosed with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer treated with fulvestrant in second-line or beyond.
With a large number of patients and prolonged follow-up, the study meets its objectives by providing valuable insight into outcomes, patient prognosis, and survival in routine clinical practice.
The results obtained were somewhat lower than those reported in pivotal studies, highlighting the importance of local research to guide clinical practice and health policy. Liver and bone metastases were associated with worse prognosis and survival.
It is crucial to continue local studies to improve treatment strategies according to the specific context. In addition, health policies that ensure efficient management of financial resources should be implemented, especially in low- and middle-income countries, to improve treatment effectiveness and resource management in healthcare.

