Estudios originales
← vista completaPublicado el 21 de julio de 2025 | http://doi.org/10.5867/medwave.2025.06.3002
Púrpura trombótica trombocitopénica: descripción y análisis de 23 casos tratados en Chile entre 2017 y 2022
Thrombotic thrombocytopenic purpura: Description and analysis of 23 cases treated in Chile between 2017 and 2022
Abstract
Introduction Since the first description of Thrombotic Thrombocytopenic Purpura, caused by a severe deficiency of ADAMTS13, plasma exchange and immunosuppression have become standard treatments, allowing to decrease its high mortality rate. Prospective records of Thrombotic Thrombocytopenic Purpura have provided valuable information on its pathophysiology, clinical presentation, and outcomes. The objective of this study is to update the local Chilean experience in the diagnosis and management of this disease, through a case series of patients treated between 2017 and 2022.
Methods Case series study that included patients over 18 years old diagnosed with Thrombotic Thrombocytopenic Purpura, treated between June 2017 and August 2022 at Hospital Clínico UC Christus. Information was collected from clinical records, which were used for cohort description and statistical analysis. Accepted definitions from the literature were used to describe the outcomes. The study was approved by the local ethics committee (ID 220524001).
Results Our series had higher age and prevalence of comorbidities compared to those reported in the literature. The most important clinical manifestations included constitutional, gastrointestinal, hemorrhagic, and neurological symptoms, with different presentation frequencies than those described internationally. We found a lower capacity of the PLASMIC Score for the detection of Thrombotic Thrombocytopenic Purpura in our series. The predominant therapeutic strategy was a combination of glucocorticoids and plasma exchange (61% of the patients). There was a high mortality rate (56.5%) and adverse events related to plasma exchange, especially of infections related to its use.
Conclusions This study highlights the diagnostic and therapeutic challenges of Thrombotic Thrombocytopenic Purpura in the local context and the need to improve our management strategies through standardizing care and better application of clinical guidelines to reduce the high mortality rate in these patients.
Main messages
- Thrombotic thrombocytopenic purpura is a rare and highly lethal disease.
- Our series presents data on the Chilean experience in managing this disease.
- Thrombotic thrombocytopenic purpura is a rare and highly lethal disease.
Introduction
Since the initial description of thrombotic thrombocytopenic purpura by Eli Moschcowitz in 1924, characterized by high mortality, the understanding of the disease has progressed significantly [1,2,3]. The introduction of plasma exchange in the 1970s marked a milestone, notably reducing its lethality [1,2,4,5,6,7]. Subsequently, the incorporation of immunosuppressive treatments such as glucocorticoids, rituximab, and, more recently, caplacizumab, has further refined the therapeutic strategy, reducing mortality by 10 to 20% [8,9].
Thrombotic thrombocytopenic purpura is characterized by a severe deficiency of the protease ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13), which is essential for regulating von Willebrand factor multimers. In its congenital form, known as Upshaw-Schulman syndrome, or in the acquired variant, mediated by inhibitory autoantibodies, this deficiency leads to the accumulation of large Von Willebrand factor multimers. These promote abnormal platelet aggregation and thrombus formation in the microcirculation, triggering thrombotic microangiopathy and ischemic tissue damage. This provides insight into the manifestations of the disease and the diagnostic criteria that define it, including the presence of microangiopathic hemolytic anemia, thrombocytopenia, and tissue damage in the context of a severe deficit in ADAMTS13 activity (less than 10%) [1,2,9,10].
Initially, thrombotic thrombocytopenic purpura was defined by a "classic pentad" of symptoms including microangiopathic hemolytic anemia, thrombocytopenia, fever, neurological symptoms, and renal failure. However, more recent cohorts have shown that this presentation is very rare, occurring in less than 10% of cases. It is now recognized that thrombotic thrombocytopenic purpura can manifest in a wide variety of ways, creating challenges in its early and accurate diagnosis [11,12,13,14,15,16,17]. The modern diagnosis of this pathology is based on the demonstration of ADAMTS13 activity of less than 10%, which has a specificity of more than 97% when applied in the appropriate clinical context. Despite its diagnostic utility, measurement of ADAMTS13 activity is not widely available. This situation can lead to delays in the diagnosis and treatment of thrombotic thrombocytopenic purpura [2,18]. To address this issue, tools such as the PLASMIC score, which utilizes clinical history and laboratory variables, have been developed to predict thrombotic thrombocytopenic purpura in suspected cases, enabling early therapeutic measures [19,20].
Given the rarity of thrombotic thrombocytopenic purpura, prospective registries have been crucial in accumulating information on its pathophysiology, clinical presentation, and long-term outcomes. The Oklahoma group pioneered the first systematic registry of patients with thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. This model has inspired similar initiatives in several countries, including the United Kingdom, Australia, New Zealand, Japan, Korea, Italy, Spain, and France. These international registries have enabled the overcoming of limitations in single-center studies and the comparison of data from different populations, thereby enriching our understanding of the disease [1,12,13,14,15,16,21,22].
At the national level, thrombotic thrombocytopenic purpura was addressed in a retrospective series of 18 cases attended at the Hospital Clínico de la Red de Salud UC-Christus, a university center based in Chile, published by Eymin et al. in 2008 [23]. Since then, significant advances have been observed in both the diagnosis and management of this disease. In particular, the measurement of ADAMTS13 activity has been consolidated as a diagnostic standard in clinical practice. In therapeutic terms, in addition to plasma exchange and systemic corticosteroids, the role of other adjuvant or second-line therapies, including rituximab and caplacizumab, has been clarified.
This study aims to update the local experience regarding the diagnosis and management of thrombotic thrombocytopenic purpura, using the records of cases treated at the Clinical Hospital of the UC-Christus Health Network between 2017 and 2022. For this purpose, baseline clinical characteristics, therapeutic interventions employed, and observed outcomes are described and compared. In addition, possible associations are explored between PLASMIC score and patient comorbidity burden (quantified using the Charlson comorbidity index), between clinical presentation variables and mortality, between different treatments and mortality, and between adverse plasma exchange events and mortality. A characterization of the clinical picture at presentation, the therapeutic measures used, and the outcomes observed in this series are presented below.
Methods
This is a case series study (retrospective descriptive design) involving cases of thrombotic thrombocytopenic purpura treated at the Clinical Hospital of the UC-Christus Health Network between 2017 and 2022. All patients over 18 years of age, with a severe deficit in ADAMTS13 activity (i.e., less than or equal to 10%), who were seen between June 8, 2017 and August 23, 2022, at the Hospital Clínico de la Red de Salud UC-Christus (hereafter, the hospital) were included within the series. Information was collected from clinical records, including demographic variables, clinical manifestations, laboratory parameters, treatment modalities, and outcomes. The data were anonymized by coding when included in the database. Hospitalization times were considered from admission to any tertiary center to the time of discharge from the hospital. For follow-up data, only those patients for whom clinical data could be obtained from the clinical record and national public registries were considered.
The PLASMIC score (a tool for predicting the diagnosis of thrombotic thrombocytopenic purpura) and the Charlson comorbidity index (a comorbidity burden scoring system associated with 10-year survival for each patient) were calculated using the available information up to the time of disease suspicion [20,24,25]. For the description of outcomes, we used the definitions currently accepted in the literature, which are included in Table 1 [9].
In case of missing data, a pairwise deletion approach was used, leaving the respective data as missing. For the analysis, those observations with complete data on the variables of interest were included, which may vary the sample size (n) for each variable. In this way, neither the complete variable is eliminated nor the entire case is discarded, but only the missing observation for the corresponding analysis.
ADAMTS13 activity was measured using the fluorescence resonance energy transfer method (FRETS), with the use of the fluorescence quenching substrate for ADAMTS-13 (FRETS-VW73, Peptide Institute, JP), calibrated against the first WHO International ADAMTS-13 Plasma Standard, Plasma NIBSC 12/25. The search for an inhibitor was performed by mixing the test with the plasma pool, with any result equal to or greater than 0.7 Bethesda units per milliliter being considered positive.
Statistical analysis
For the description of the baseline characteristics of the study population and their analysis, different statistical methods were used depending on the type and distribution of the data. Continuous variables were presented as mean plus/minus standard deviation (SD) for those with a normal distribution, or as median accompanied by the interquartile range (IQR) in cases where this distribution applied. On the other hand, categorical variables were described in terms of both absolute and relative frequencies (percentages). The normality of the numerical variables was assessed using the Shapiro-Wilk test. Comparison between normally distributed continuous variables was performed using Student’s t-test, while the Kruskal-Wallis test was used for those without normal distribution. To examine the relationship between categorical variables, Pearson’s Chi-square test or Fisher’s exact test was used, depending on the characteristics of the variables. Differences between numerical variables were analyzed using the Student’s t-test or the Mann-Whitney test, depending on their distribution (normal or non-normal, respectively).
To investigate a possible association between the PLASMIC score and clinical variables, including age, individual comorbidities, and the Charlson comorbidity index, Spearman’s Rho (ρ, a nonparametric correlation coefficient measuring the strength and direction of the association) and logistic regression analysis were employed.
For all analyses, a value of p < 0.05 was set as the threshold for statistical significance. All statistical analyses were performed using STATA software, version 17.0. This case series has been reported under the Preferred Reporting of Case Series in Surgery (PROCESS) guideline [26].
The study was approved by the local ethics committee ID 220524001, dated March 30, 2023. Given the retrospective nature of the research and the use of existing clinical data, a waiver of informed consent for chart review was obtained.
Results
During the described period, 23 adult patients with severe ADAMTS13 deficiency were treated at our institution. All of them with the presence of inhibitory antibodies against ADAMTS13. Of these, one patient had incomplete clinical records; therefore, only the data available for this patient were included in the analysis (as previously described, missing data were excluded from the analysis). Seven patients were referred to our hospital from other centers, where they had their initial consultation. Additionally, one patient was transferred to another center after the initial consultation at our hospital, so we did not have access to information on his clinical progress after the transfer. Table 2 shows a summary of the 23 cases, together with their most relevant demographic and clinical variables at the time of the suspected diagnosis of thrombotic thrombocytopenic purpura.
Clinical and laboratory data at the time of clinical presentation are presented in Table 3. Of the 23 patients included in the series, 91% (21) were admitted to a monitored unit, including 30.4% (7) to the intensive care unit.
PLASMIC score and association with clinical variables
The median PLASMIC score was 5 (interquartile range 4 to 5.5), 70% had a score equal to or higher than five at the time of suspicion, and 26.1% had a PLASMIC score equal to or higher than 6. As previously described, the correlation between the PLASMIC score and various clinical variables, including age and the burden of comorbidities, was evaluated, both concerning specific diseases and the overall disease burden. For this purpose, the Charlson Index was used, with cutoff values for the PLASMIC score set at both 5 and 6 or higher. Although no association was found between isolated comorbidities and a lower PLASMIC score, a trend towards a lower PLASMIC score with a higher burden of comorbidities was observed. This was supported by an Odds ratio of 0.74 for PLASMIC less than 5, although it did not achieve statistical significance by logistic regression (p = 0.059, 95% confidence interval: 0.54 to 1.01).
Therapies used and outcomes
In our cohort, the predominant therapeutic strategy was the combination of glucocorticoids and plasma exchange in 61% of patients (14). All of them were treated with high or very high prednisone equivalent doses, and 11 of them (48%) received pulse doses (equivalent doses equal to or greater than 125 milligrams of methylprednisolone per day) at the beginning of treatment. The therapies, treatment strategies used, and outcomes are summarized and schematized in Table 4 and Figure 1.
Treatments used and hospital survival in the cohort described.
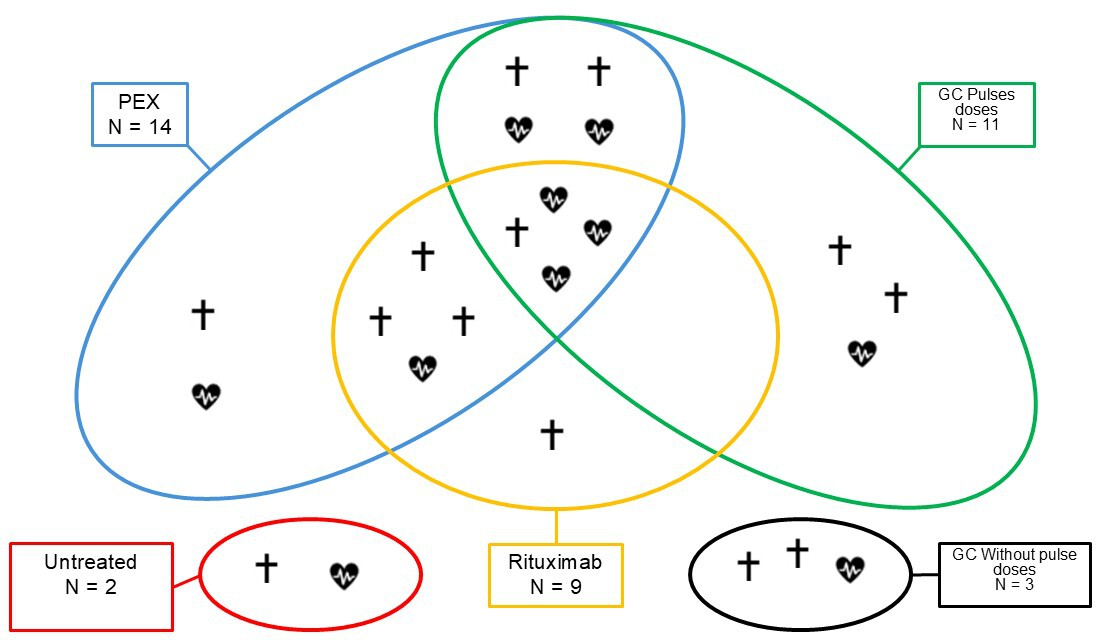
Source: Prepared by the authors of this study.
A significant association was found between lactate dehydrogenase levels greater than three times the upper limit of normal and mortality, as well as lower mortality in patients with hemorrhagic manifestations (p < 0.05).
Of the total number of patients who received plasma exchange (n = 14), all experienced some adverse effect: 6 were mild and 8 were severe (43% and 57%, respectively). Among the mild cases, there were two instances of paresthesia, one case of hypocalcemia, and three cases of isolated febrile reactions. On the other hand, severe adverse effects included four patients with severe hypotension and need for vasoactive drugs, two with volume overload, one with acute lung injury, and one with subarachnoid hemorrhage. Seven of these patients (87%) died during their hospital stay, while none of those who experienced mild adverse effects died, showing a statistically significant association between presenting severe adverse effects during plasma exchange and mortality (p = 0.001). Seventeen patients (74%) had in-hospital infections, which were statistically significantly associated with the use of plasma exchange (p = 0.021), but not with the use of pulsed glucocorticoids or rituximab. Of these, 10 (59%) died during their stay versus 3 (50%) of the group that did not present infections. In-hospital mortality was 56.5%, directly or indirectly attributable to thrombotic thrombocytopenic purpura in 92% of those who died. Additionally, only seven patients achieved a clinical response during treatment, and of these, three experienced exacerbations.
Of the 10 patients who survived the hospital stay, a median follow-up of 719 days (interquartile range, 515 to 841 days) is available. One of the patients was discharged to another hospital prior to the diagnosis of thrombotic thrombocytopenic purpura and died within 30 days of discharge. This group presented two cases of clinical recurrence, one 47 days after response was achieved and the other at 1311 days.
An illustrative case of the clinical evolution of patients with thrombotic thrombocytopenic purpura in our case series is that of a patient with a history of systemic lupus erythematosus and antiphospholipid syndrome who presented 12 hours of respiratory symptoms and progressive compromise of consciousness. She was admitted to the emergency department of another hospital with severe respiratory failure, hypotension, and poor perfusion. She was intubated, stabilized, and transferred to our hospital due to the need for a critical bed. The neurological examination on admission showed right hemiplegia and aphasia. CBC with smear showed anemia (Hb 5.2), thrombocytopenia (PLT 39 000), and schistocytes (++). PLASMIC score at the time of suspicion was 5 points. Antiphospholipid serology was triple positive with high titers of immunoglobulin G antibodies against cardiolipins and against B2-glycoprotein I. ADAMTS13 activity was less than 10% and the presence of an inhibitor was detected. Computed tomography of the brain showed signs compatible with an acute cerebral vascular accident in the left middle cerebral artery, and computed angiotomography of the chest showed signs of bilateral acute pulmonary thromboembolism. Cases such as this one highlight the diagnostic challenges that can arise in cases of thrombotic microangiopathy. In particular, this case met both the diagnostic criteria for thrombotic thrombocytopenic purpura and catastrophic antiphospholipid syndrome, and it is impossible to rule out some level of overlap between the two entities. Pathophysiologically, both are autoimmune diseases mediated mainly by antibodies. Although the patient started anticoagulation, received pulse corticosteroids and plasmapheresis on the same day of admission to our center, she evolved with volume overload and acute pulmonary edema, forcing us to suspend therapy. In the following hours, he developed progressive multiple organ failure and died 48 hours after admission.
Discussion
This paper provides an updated local perspective on the presentation and management of patients with thrombotic thrombocytopenic purpura in Chile, building on the foundation laid by Eymin et al in 2008 [23]. Our series of patients presented distinctive clinical and demographic characteristics compared to previously published international series. Patients were older (median 62 years) and had a high prevalence of significant comorbidities, including hematologic malignancies (17.4%) and chronic kidney disease (26%). Clinically, presentation with constitutional (86.4%), gastrointestinal (52.2%), and neurological (34.8%) symptoms predominated, with a lower frequency of severe neurological symptoms compared to other cohorts. The PLASMIC score demonstrated reduced sensitivity in our population, which may be related to the higher prevalence of comorbidities. In therapeutic terms, 61% of patients received plasma exchange combined with glucocorticoids, but the in-hospital mortality rate was high (56.5%). This was associated with both nosocomial infections and serious adverse events related to plasma exchange. Some of these findings are discussed in depth below.
Our cohort is characterized by a mean age well above 50 years, in contrast to the younger age observed in international registries. This older age is accompanied by a high prevalence of comorbidities, particularly hematologic malignancies and chronic kidney disease. Regarding clinical manifestations, we observed a significantly higher incidence of gastrointestinal symptoms (greater than 50%) and a lower prevalence of neurological symptoms (35%) compared to national (83%, as reported by Eymin et al.) and international series (greater than 50%) [12,13,14,15,23,27,28].
Continuing with the diagnosis, we would first like to note differences concerning the previous series published by Eymin’s team. In it, not all patients had ADAMTS13 activity below 10%, reflecting a change in diagnostic criteria and strategies in recent years. Still, it is possible that patients with ADAMTS13 equal to or greater than 10% represent atypical variants of thrombotic thrombocytopenic purpura, as described by George et al [17]. Currently, thrombotic thrombocytopenic purpura remains a diagnostic challenge, with an average of 32 days from admission to clinical suspicion in our series. However, no significant delay was found between clinical suspicion and the request for ADAMTS13 activity testing (median, 1 day). Using the PLASMIC score, we found a lower ability to detect thrombotic thrombocytopenic purpura compared to what is reported in the literature. Using a cutoff score equal to or higher than 5, 70% of patients were screened, as opposed to 97% and 99% reported by Bendapudi et al. and Paydary et al., respectively [20,24]. These differences may be due to the high prevalence of chronic kidney disease and the higher median age in our series (62 years) compared to that of Bendapudi (47 years). This phenomenon is illustrated by the potential association observed between a higher Charlson index and lower PLASMIC scores. However, we failed to establish a statistically significant correlation between these variables. This aspect could be attributed to a limitation in the statistical power of our study, due to its small sample size. In the absence of another predictive score, we consider the PLASMIC to remain a useful tool in the Chilean population. However, further studies are required to validate it in the local context.
In therapeutic terms, the utilization of plasma exchange in our cohort was 61%, which is considerably lower than the rate reported worldwide (over 90%). The use of corticosteroids was concordant with the 91% reported internationally, while rituximab was administered in 40% of cases. No patient was treated with caplacizumab, not yet approved in Chile [12,15,29]. The case-by-case analysis revealed that the omission of plasma exchange was due to the severity of the patients and to decisions of therapeutic proportionality. However, situations were identified in which plasma exchange was not used, despite the adoption of intensive therapeutic measures. This suggests a lack of standardization in the treatment of thrombotic thrombocytopenic purpura and a divergence from the recommendations provided by clinical guidelines [29].
Consequently, it is essential to address the high in-hospital case fatality rate in our series, which exceeded 50%, notably higher than the 10-20% range documented in the literature [8,30]. This high rate could be partially influenced by the higher age and prevalence of comorbidities, as well as by other critical factors, such as low utilization of plasma exchange and rituximab, a limited therapeutic response (with only 7 patients achieving a platelet count above 150 000), and a high incidence of nosocomial infections. The latter and their associated mortality are possibly linked to the use of plasma exchange due to the need for central venous access, as previously described. The lack of association with mortality of the different therapies is likely also explained by the limited statistical power of our study. Therefore, these results may not be representative of other cohorts or populations. Other clinical factors associated with higher mortality in our cohort were lactate dehydrogenase levels at admission, above three times the upper limit of normal, and the absence of hemorrhagic symptoms at the time of suspicion. The latter probably explains the delay in diagnosis. The clinical case described in the results section exemplifies how challenging it is to confront, diagnose, and treat this group of patients, as not every patient fits the disease descriptions found in the literature.
Our study presents several significant strengths, including the ample amount of data available for the evaluation of each case and the detailed characterization of the diagnosis and treatment of these patients within the local context, which highlights the diagnostic and therapeutic challenges involved in this disease in our country. However, we face notable limitations: the small size of the cohort, the retrospective nature of the investigation, and the challenges associated with searching non-digitized records. In addition, the loss of data and the complexity of interpreting the available information have been considerable obstacles. This was particularly evident in the interpretation of cases with low ADAMTS13 levels but no formal diagnosis of thrombotic thrombocytopenic purpura during hospitalization. All these factors ultimately indicate that the series of patients presented, along with their clinical characteristics and outcomes, is neither representative nor applicable to other populations.
Despite the above, our study allows us to outline an interesting corollary: the PLASMIC score may not apply to patients or populations with a high burden of comorbidities, thereby diminishing its performance. This may hinder the diagnosis and initiation of early therapies in patients who present with thrombotic thrombocytopenic purpura. We believe that following the example of the international series cited above and implementing local prospective databases would be beneficial for studying this disease in order to propose explanations and solutions to the diagnostic and therapeutic conflicts raised in our work. Specifically, the validation of the PLASMIC score in our population, to clarify its association with comorbidities and to standardize the therapeutic measures used for its treatment.
Conclusiones
Thrombotic thrombocytopenic purpura remains a diagnostic and therapeutic challenge, with a high mortality rate.
Although our series provides valuable data for a better characterization of thrombotic thrombocytopenic purpura in Chile, prospective studies are imperative. Ideally, these should be implemented on a national scale to validate diagnostic support tools, such as the PLASMIC score, and standardize therapeutic interventions. Such an effort will help us to optimize the diagnosis and treatment of thrombotic thrombocytopenic purpura, with the primary objective of reducing mortality.

