Estudios originales
← vista completaPublicado el 19 de junio de 2025 | http://doi.org/10.5867/medwave.2025.05.3060
Adaptación y validación de expertos de un instrumento de autopsias verbales en Chile utilizando el método Delphi
Expert adaptation and validation of a verbal autopsy instrument in Chile using the Delphi method
Abstract
Background In Chile, despite high obstetric coverage and consolidated registration systems, there has been a stagnation in the reduction of maternal mortality, reflecting the need to identify social factors and determinants. These are often overlooked by traditional surveillance systems.
Objective To adapt and validate, through expert consensus, a verbal autopsy instrument based on the three delays model, with a gender and intercultural approach.
Methods Expert validation study using the Delphi method in three phases, carried out between June and October 2024. Forty experts in maternal health, epidemiological surveillance, gender, and interculturality participated. Seven criteria were evaluated: clarity, relevance, cultural relevance, socio-health relevance, regulatory relevance, and the incorporation of gender and intercultural approaches. The process sought to reach consensus to ensure the methodological and contextual quality of the instrument.
Results The final instrument includes 95 items organized into six thematic blocks. An overall consensus of 85% was achieved on the evaluated criteria. The adaptation incorporated variables such as mental health, gender-based violence, ethnic identity, and perceived quality of care. Operational validation identified implementation, logistical, and ethical challenges, leading to a gradual implementation plan in regulatory, pilot, and national expansion phases.
Conclusions The instrument adapted and validated by experts offers a complementary tool for monitoring maternal mortality in Chile. Its comprehensive approach would allow identifying social and structural factors associated with maternal deaths, favoring more equitable and culturally relevant interventions aimed at preventing avoidable maternal deaths. Field validation is essential to assess the impact of its application.
Main messages
- Maternal mortality in Chile faces significant challenges, particularly with sociocultural factors, which traditional surveillance systems fail to capture.
- This study adapts and validates, through expert review, a verbal autopsy instrument using the Delphi method, integrating gender and intercultural approaches for a more contextualized analysis.
- The study is limited to the process of adaptation and expert validation of the instrument, without including its field validation. However, this is planned as a future step.
- An expert-validated instrument was developed to identify gaps in healthcare services and structural factors, providing a replicable tool to improve maternal mortality surveillance in similar contexts.
Introduction
Maternal mortality, a sensitive indicator of gender inequality, remains a public health priority [1]. Despite global progress, regional disparities persist: Africa continues to have the highest maternal mortality ratio, Asia is showing progress, while in the Americas and Europe, the maternal mortality ratio has increased between 2000 and 2020. In Latin America, this setback was exacerbated by the COVID-19 pandemic, which deepened barriers to timely and quality care [2].
Maternal mortality is a multi-causal phenomenon, and understanding and analyzing its causes is essential for designing policies and interventions that effectively respond to women’s real needs [3,4,5]. In this regard, current recommendations for maternal mortality surveillance and response promote systematic case review to identify modifiable factors that contribute to preventable deaths and guide preventive strategies [6,7]. In this scenario, audits have proven to be effective tools for improving health outcomes. However, their impact may be limited if they are not analyzed considering the context in which the deaths occur [8,9].
The context of Chile and the need for a comprehensive approach
Chile is in an advanced stage of obstetric transition, with high coverage rates, low maternal mortality ratios, and a consolidated system of statistical records and clinical audits [10]. However, in recent years, there has been a stagnation in the reduction of maternal mortality ratios, along with an increase in maternal deaths from indirect and late causes. During the COVID-19 pandemic, the latter reached an excess mortality rate of 104% [11]. Under these circumstances, cases classified as “other obstetric conditions not classified elsewhere” predominate [12]. This suggests not only limitations in diagnostic accuracy but also inadequacies in current registries that prevent them from adequately capturing emerging issues, which limit sustained progress in reducing maternal mortality. While countries in the early stages of transition, with high maternal mortality ratios, prioritize their efforts to improve access to and coverage of obstetric care to reduce these deaths, countries with advanced health systems face challenges with maternal mortality that go beyond the availability of obstetric services [13]. These require a comprehensive approach, which involves identifying and addressing all the factors that influence their origin and development, articulating the various dimensions (biological, psychological, social, cultural, economic, environmental, ethical, among others) to understand the complexity of the problem and propose more effective interventions to accelerate sustainable mortality reduction [3]. This involves not only improving the quality of services and reducing delays in care, but also identifying and addressing gender inequalities and social and intercultural factors that contribute to delays, particularly in vulnerable populations [7].
The need for a verbal autopsy instrument in Chile
Verbal autopsies are mortality surveillance tools consisting of structured interviews with family members or witnesses of the death. Their purpose is to collect information on previous symptoms, access to medical care, and circumstances of death. This allows for improved identification of the underlying cause of death [14]. Their use has been documented mainly in contexts where registration systems are limited [15] and in countries with high rates of community deaths [16]. However, previous experiences have demonstrated their applicability in other settings. One example is the Intentional Search and Reclassification of Maternal Deaths (BIRMM) system, which incorporates verbal autopsy to identify underestimated or misclassified cases of maternal mortality [17]. With this system in Argentina, a 2014 study revealed that 4.95% of maternal deaths had not been correctly recorded [18], while in Mexico, it allowed for the reclassification of 932 additional deaths [19].
Verbal autopsy is thus proposed as a tool that can strengthen mortality surveillance, depending on the needs of each country [20]. In Chile, it could contribute to a more comprehensive interpretation of the data obtained from death certificates and statistical records. This would not only make it possible to specify the causes of death, but also to deepen the analysis of social determinants such as gender and migration, as well as experiences related to access to and quality of services, and sociocultural inequalities. Its adaptation to the national context would complement existing surveillance systems and enhance the identification of factors that continue to impact maternal health.
During the pandemic, the United Nations Population Fund for Latin America and the Caribbean (UNFPA-LACRO) conducted a study in three Latin American countries, including Chile, using a verbal autopsy tool based on the three delays model [21]. This model aims to identify and analyze the impact of delays in the decision to seek care (first delay), access to healthcare services (second delay), and the provision of adequate care (third delay) on maternal mortality [22]. The results highlighted the need to consider social, cultural, and intersectional factors to gain a better understanding of the inequalities that determine maternal mortality in Latin America.
Objective of the study
To adapt and validate, through expert review, a verbal autopsy instrument based on the three delays model, with a gender and intercultural approach. The adaptation and validation also seek to ensure its relevance in the Chilean social, healthcare, and regulatory context, guaranteeing its applicability before implementation in the field.
Methods
Methodological study with a mixed (qualitative and quantitative) approach, sequential adaptation, and expert validation of a verbal autopsy instrument for maternal mortality, carried out using the three-phase Delphi method [23,24]. This method was chosen for its ability to identify consensus on issues with limited evidence and to adapt tools to new sociocultural contexts [25]. The adaptation of the instrument involved modifying the language and content to ensure its clarity, as well as cultural, socio-health, and regulatory relevance, and to incorporate a gender and intercultural approach [23]. Expert validation referred to the process in which specialists evaluated the instrument to determine its clarity, relevance, and appropriateness before its validation in the field [23]. This validation will be addressed in future studies, allowing for the evaluation of its applicability in real-life settings and its final impact on maternal mortality surveillance.
Instrument evaluation criteria
To guide the adaptation and validation, seven criteria were established, as shown in Table 1.
Description of the instrument
The original verbal autopsy instrument used corresponds to an interview guideline based on the three delays in the maternal mortality model [22]. It consists of 88 questions organized into six blocks designed to collect contextualized information on the causes of maternal death in the context of COVID-19 [21].
As shown in Table 2, the instrument addresses sociodemographic data, health history, and factors associated with each of the three delays, in addition to perinatal aspects. Adaptation and validation by experts did not modify the overall structure, number, or nomenclature of the blocks. The final version of the instrument is available upon request from the authors.
Selection and profile of experts
Experts were selected through purposive sampling [26], ensuring disciplinary, regional, and institutional diversity to minimize bias and ensure a comprehensive evaluation of the instrument. Priority was given to professionals with experience in maternal health, epidemiological surveillance, gender, interculturality, and public policy. Representatives from academia, government ministries, and international organizations were included. To mitigate the risk of group bias, experts from diverse regions of the country were included, and two of the three phases of the Delphi process were conducted anonymously. This allowed for independent evaluations and ensured that adjustments were based on consensus rather than dominant individual opinions.
The first phase, focused on adapting the instrument, involved experts with experience in verbal autopsy, maternal health, gender, and intercultural issues. In the second phase, focused on validating clarity, relevance, and appropriateness, specialists in maternal health and epidemiological surveillance were convened. To ensure that the instrument integrated an intersectional perspective, priority was given to the inclusion of specialists in gender studies and public health. In addition, experts in interculturality were incorporated.
In the third phase, aimed at operational validation, decision-makers with experience in implementing maternal health strategies and epidemiological surveillance participated, prioritizing profiles with leadership in public policy and maternal and child healthcare program management.
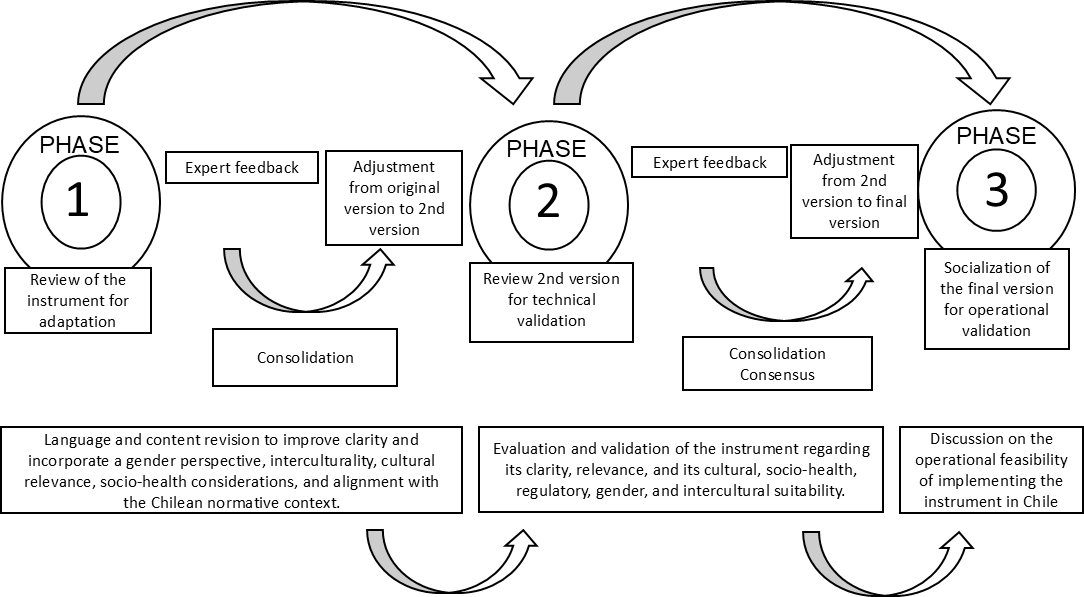
Adaptation and validation process with experts: information gathering and adjustment of the instrument using the Delphi method
The three phases of the Delphi method were conducted between June and October 2024, within the framework of a collaboration between UNFPA-LACRO and the Department of Women’s and Newborn Health Promotion at the University of Chile, to which the research team is affiliated.
Experts were invited to participate in each phase via personalized email invitations. These invitations detailed the purpose of the study, the procedure for adapting and validating the instrument, and the type of participation required. All participants gave their informed consent before each phase, ensuring the confidentiality and voluntary nature of the process.
In the first phase, participants conducted a thorough review of the original version of the instrument. Using an electronic form, they proposed adjustments to the language and content to enhance its clarity, cultural relevance, regulatory compliance, and social and health relevance, and to incorporate gender and intercultural approaches. The qualitative feedback obtained was consolidated by the research team and used to develop a second version of the instrument, which was refined based on the contributions received.
In the second phase, an expanded panel of experts evaluated the second version, completing an electronic form in which they rated the assessment criteria using a Likert scale from one to five, with one representing “strongly disagree” and five representing “strongly agree.” Additionally, open-ended questions were included to gather qualitative observations that would facilitate further adjustments. The gathered information in this phase was systematized and consolidated by the research team, prioritizing adjustments based on the level of consensus achieved.
In the third phase, a final version of the instrument was consolidated, incorporating the changes derived from the previous phases. This version was presented and discussed in a virtual meeting with a final panel of experts, who, through open-ended questions, debated the requirements necessary for its implementation in Chile. This discussion provided expert insight into the operational feasibility of the instrument in the national context, addressing challenges and strategies for its application.
This process ensured that each version of the instrument reflected the contributions of the experts, guaranteeing its methodological consistency and applicability in maternal surveillance in Chile (Figure 1).
Delphi phases in the process of adaptation and expert validation of the verbal autopsy instrument in Chile.
Analysis
In the first phase, a deductive thematic content analysis was conducted, seeking to identify previously established categories within the text. These are constructed using theoretical references [27], in this case based on the instrument’s evaluation criteria. This allowed us to consolidate the required changes, identify the relevance of items, adjust the language, and incorporate or modify items to carry out the adaptation.
In the second phase, the same qualitative analysis was applied to the open-ended questions, while the quantitative data were analyzed using descriptive statistics. The “agree” averages were calculated according to the Likert scale scores, as well as the consensus percentages, defined as the proportion of experts who ‘agreed’ or “strongly agreed” that the instrument met the criteria evaluated. For the validation of each criterion, a minimum threshold of 75% consensus was established, considered adequate for qualitative instruments in under-explored areas [25].
The overall consensus on the instrument’s validation was determined by averaging the levels of agreement obtained for each criterion evaluated. This, based on the Delphi method, enables the synthesis of the overall degree of acceptance of the instrument and has been used in previous studies to validate health tools [28].
In the third phase, the discussion panel was recorded and transcribed. In this case, an inductive thematic content analysis was performed, characterized by the construction of categories emerging from the content [27]. This allowed the identification of the requirements, needs, or challenges considered by the panel for the instrument´s field implementation.
Results
This section provides the results obtained during the Delphi phases, following the characterization of the participants.
A total of 40 experts participated, of whom 29 (73%) were women. Their distribution by phase and profile is shown in Table 3.
First phase
After analyzing and consolidating the feedback from this phase, 12 questions were reformulated to improve clarity, with their wording optimized and inclusive and accessible language incorporated. In terms of social and health relevance, questions related to COVID-19 were removed, and new items were added to reflect Chile’s current epidemiological profile, with a focus on identifying late maternal mortality and indirect causes of death.
In terms of regulatory relevance, questions were included to assess compliance with national maternal healthcare regulations and identify regulatory barriers to access to care, with an emphasis on vulnerable populations, including migrant and indigenous women. From a gender perspective, questions were added on reproductive autonomy, gender-based violence, and barriers to care, addressing experiences of treatment and care in services that could affect the decision to seek care. Finally, in the intercultural dimension, questions were added on ethnic self-identification, language barriers, and respect for cultural beliefs. This made it possible to highlight inequalities in the provision of health services. As a result of these adjustments, the total number of items increased from 88 to 91, strengthening the instrument’s ability to capture the structural determinants of maternal mortality in Chile. The main changes implemented in this phase are detailed in Table 4.
Second phase
Expert validation in this phase demonstrated the level of consensus achieved on each of the established criteria, allowing the instrument to be adjusted in line with the findings obtained.
In terms of clarity, 85% of experts considered the items to be understandable. However, technical terms and lengthy wording were identified, prompting adjustments to improve fluency and avoid redundancy.
Regarding relevance, 90% of the participants validated the instrument’s ability to address the determinants of maternal mortality, highlighting the usefulness of the three delays model and its intersectional approach. This strengthened the integration of sociocultural, economic, and gender factors.
A cultural relevance achieved 92% consensus, with an emphasis on incorporating cultural practices, beliefs related to maternal health, and ethnic self-identification. All these elements are integrated into the sociodemographic block.
Regarding sociosanitary relevance, 80% agreed or strongly agreed on its adequacy, recommending that the instrument’s scope be broadened to include mental health, substance use, and physical activity. These elements were integrated into the obstetric and general health history block, allowing for a more holistic approach.
Regulatory relevance also obtained 80% consensus. Alignment with national protocols was reinforced through questions that identified structural barriers, particularly in equitable access to care and the fulfillment of reproductive rights.
The gender approach was validated by 75% of experts. It was suggested that aspects of discrimination, perception of symptom severity, and specific barriers affecting women be explored in greater depth. These adjustments were reflected in the first and third delay blocks.
Finally, the intercultural approach achieved 88% validation, highlighting the incorporation of questions on ethnic and linguistic diversity. Here, the understanding of how cultural differences influence the experience and access to maternal care was reinforced.
Consensus on the instrument’s validation
The second phase of the instrument validation process achieved an overall consensus of 85%, reflecting a high level of agreement among experts regarding its clarity, relevance, and appropriateness. This consolidated acceptance of the instrument as a methodologically appropriate tool to complement maternal mortality surveillance in Chile.
After the second phase, the instrument went from 91 to 95 items, distributed across the same six blocks. The main modifications are presented in Table 4.
Third phase
The analysis of this phase identified three categories of challenges for implementing the instrument and developing an implementation plan.
Challenges in the application
The need for training for interviewers was highlighted, focusing on cultural and gender sensitivity, as well as psychological first aid. This is to avoid bias in data collection and to provide an initial response to emotional crises experienced by interviewees. The importance of developing a detailed application manual was emphasized to standardize the conduct of interviews and minimize variability in data collection.
Challenges in logistics implementation
Difficulties in accessing informants were identified. This is particularly significant in rural areas and indigenous communities, where language barriers or mobility difficulties could limit implementation. It was recommended that the use of digital technologies be explored to facilitate information collection in hard-to-reach areas. Additionally, the need to consider the budget for implementation was emphasized. A phased implementation strategy was proposed, starting in regions with higher maternal mortality rates to assess the feasibility of the process before national expansion.
Ethical and confidentiality challenges
The importance of ensuring the confidentiality of information and protecting collected data was emphasized, especially in contexts where families may experience situations of vulnerability. The development of emotional support protocols for interviewers and participants was suggested, given the sensitive nature of the interviews.
Based on these findings, strategies emerged to address these challenges for field implementation.
These include the creation of a three-stage implementation plan (Table 5), accompanied by a monitoring and evaluation system to allow for adjustments before national scaling up. Additionally, the creation of a supervisory committee was recommended to ensure the quality and integrity of the data collection process.
Discussion
Although there are experiences that have incorporated verbal autopsies in countries with weak surveillance systems [29,30], their impact on healthcare systems with consolidated statistical records has been scarcely explored. This instrument seeks to complement epidemiological surveillance in systems with consolidated records, facilitating the identification of inequalities that directly or indirectly affect maternal health and guiding more effective preventive strategies. This is consistent with the evidence supporting the incorporation of cross-cutting approaches to improve the detection of risk groups and optimize interventions [3,7].
Unlike standardized verbal autopsy instruments [31,32], this study broadens its scope by integrating structural factors and social determinants that affect maternal mortality, considering not only access to care but also the perception and quality of the service received. This perspective aligns with the most recent recommendations, which emphasize the need to address the structural inequalities that underpin maternal health [3,33]. Aspects such as migratory status, ethnic affiliation, perceived treatment in health services, and gender inequalities have been identified as factors that influence both access to and delays in care [34,35,36]. The incorporation of specific questions on these issues allows for a more comprehensive and contextualized assessment.
The instrument also includes dimensions that are usually absent from traditional surveillance systems, such as mental health, substance use, and gender-based violence. All of these variables are associated with indirect and late maternal deaths [37,38,39]. Exploring them not only facilitates more accurate classification but also promotes a better understanding of the underlying causes of these deaths.
The high level of consensus reached on the relevance and appropriateness of the instrument in all its dimensions reflects a positive assessment by experts of its usefulness in the Chilean context. The contextual application of the three delays model, together with the incorporation of cross-cutting approaches, positions this tool as an innovation in maternal mortality surveillance. This is because it broadens its scope beyond the mere determination of the cause of death [40].
However, its implementation presents significant challenges for its integration into the national surveillance system. Operational validation identified barriers, including the need for specialized training for interviewers, difficulties in accessing informants, budgetary constraints, and ethical considerations related to confidentiality, all of which are documented in the literature [20,41]. As highlighted in a global review on the incorporation of verbal autopsies into public policy, their effective implementation requires the active commitment of governments, health authorities, communities, and research teams [42].
Given the above, it is recommended that implementation is followed by a gradual plan, beginning with a phase of regulatory adjustments, followed by pilot tests in areas with high maternal mortality rates, and then a progressive expansion nationwide. This process would allow evaluating its feasibility and making modifications before its definitive integration into the maternal mortality surveillance system.
Although the tool was adapted to the Chilean context, its methodological foundations and the lessons learned from the validation process in Chile can guide other countries facing similar maternal health inequalities. Its design, based on the three delays model and the integration of gender and intercultural approaches, gives it high potential for transferability in Latin America, where intercultural and gender barriers and weaknesses in epidemiological surveillance persist [3,43]. However, its adoption requires consideration of local regulations, cultural acceptability, and institutional capacity to ensure confidentiality and an ethical approach to data collection [41]. Factors such as gender-sensitive regulatory frameworks, active maternal death review strategies, and trained teams can facilitate the implementation of this approach. Budget constraints and the limited integration of social variables into information systems represent significant challenges [42]. It is recommended that any international adoption include a participatory adaptation process to ensure technical and sociocultural relevance.
Limitations and future projections
This study focused on the adaptation and expert validation of the instrument. For this reason, field validation will be essential to assess its applicability and adjust its implementation in real-life settings. Future research should analyze its ultimate impact on maternal mortality surveillance in Chile.
Conclusions
This study presents a tool adapted and validated by experts to strengthen maternal mortality surveillance in Chile, incorporating gender and intercultural approaches. Through the Delphi method, its clarity, relevance, and operational feasibility were optimized, aligning it with the national context.
The validation of this instrument and its subsequent application in the field will generate more comprehensive and understandable information on the multiple causes that contribute to maternal mortality, including barriers to access, social conditions, and aspects of quality of care. This information is essential to guide specific interventions and public policies that comprehensively address the underlying causes of these deaths.
Specifically, this advance will directly benefit women by promoting more timely, equitable, and respectful responses from the healthcare system to prevent avoidable deaths and guarantee the right to dignified and safe care.
Operational validation enabled the design of a phased implementation plan that considers logistical, ethical, and methodological challenges, thereby facilitating its effective integration into the national surveillance system.

