Protocolo
← vista completaPublicado el 8 de mayo de 2024 | http://doi.org/10.5867/medwave.2024.04.2759
Protocolo de mapeo sistemático de intervenciones quirúrgicas y farmacológicas en el tratamiento de la neuralgia trigeminal
Protocol for a systematic mapping review of surgical and pharmacological interventions for the treatment of trigeminal neuralgia
Abstract
Introduction Trigeminal neuralgia is a painful neuropathic disorder characterized by sudden electric shock–like pain that significantly impacts patients' quality of life. Multiple treatment alternatives are available, including medical and surgical options but establishing the optimal course of action can be challenging. To enhance clinical decision-making for trigeminal neuralgia treatment, it is imperative to organize, describe and map the available systematic reviews and randomized trials. This will help identify the best treatment alternatives supported by evidence and acknowledge potential knowledge gaps where future research is needed.
Objective This systematic mapping review aims to provide up-to-date evidence on the different surgical and pharmacological treatment alternatives used for trigeminal neuralgia.
Methods A search will be systematically conducted on the Epistemonikos database to identify potentially eligible systematic reviews. Additionally, a search will be made in PubMed, CENTRAL, and EBSCO to identify randomized controlled trials assessing pharmacological and surgical treatment interventions for trigeminal neuralgia. Two independent reviewers will screen and select the studies. Data on the different treatment alternatives and reported outcomes in the included studies will be extracted using standardized forms. Following extraction, descriptive statistical methods will be used to analyze the data. The final output of this study will include an evidence map that will illustrate the connections between different treatments and their respective outcomes, providing a clear depiction of the evidence landscape.
Expected results This study expects to map, describe and assess the methodological quality of the available systematic reviews and trials on pharmacological interventions and neurosurgical procedures for treating trigeminal neuralgia. It will present the results in an evidence map that organizes the available evidence based on their different interventions and outcomes. This evidence map will serve as a visual tool to assist healthcare professionals and patients to understand evidence-based treatment options and their implications for managing this medical condition.
Main messages
- There is a lack of consensus on the best treatment options for patients with trigeminal neuralgia.
- This study aims to identify the evidence supporting pharmacological and neurosurgical interventions in trigeminal neuralgia treatment while mapping knowledge gaps.
- A graphical representation of the analyzed evidence in the form of an evidence map will help physiciansand patients select treatment alternatives supported by evidence.
Introduction
Trigeminal neuralgia is a neurological condition that affects the trigeminal nerve [1]. Classic trigeminal neuralgia arises from neurovascular compression at the convergence of the peripheral trigeminal nerve and its root. While direct interaction with arteries or veins at the root is the predominant factor, other anomalies like arteriovenous malformations, aneurysms, vestibular schwannomas, meningiomas, and various types of cysts and tumors can also induce compression. Despite the hypothesis of the direct compression of the trigeminal nerve, the comprehensive understanding of the underlying mechanism remains incomplete [2].
The pain associated with trigeminal neuralgia is recognized as one of the most intense, presenting a significant challenge in its management due to the diverse range of symptoms involved. The pain attacks endured by individuals are characterized as severe and persistent, often described as intermittent electric shock-like sensations affecting one or more divisions of the trigeminal nerve. Over time, the pain can escalate in terms of frequency, duration and intensity, reaching a point where medication alone may be insufficient [3,4].
This condition offers a range of treatment options, encompassing both medical and surgical therapies. However, the most effective treatment option has yet to be identified. The first line of treatment for all patients with trigeminal neuralgia begins with sodium channel blockers as monotherapy [5]. The most employed drugs for this purpose are carbamazepine and oxcarbazepine, which are considered the gold standard [6]. As the side effects of these drugs are a significant cause for concern [7], alternative pharmacological drugs have emerged as an option, including lamotrigine, baclofen, gabapentin, pregabalin, phenytoin, lidocaine, and sumatriptan. In some cases, alternative treatments such as botulinum toxin have also been explored [5,7]
In cases where drug treatment has proven to be ineffective or the benefits of the drugs are outweighed by their side effects, surgery becomes an alternative. Surgical interventions are classified into ablative and non-ablative procedures based on the extent of damage or destruction required for the trigeminal nerve [8]. Although surgical options are more invasive and carry the potential for irreversible complications, they are often pursued as the preferred intervention when drug treatments fail [9].
Management is often challenging, with various pharmacological and surgical alternatives described in the literature. The lack of clinical consensus is due to the broad treatment spectrum, and currently there are more than 30 pharmacological alternatives [10]. Additionally, most studies report symptomatic relief of pain rather than patient-reported outcomes. The lack of clearly defined outcomes makes comparison between treatment options challenging [11].
Randomized clinical trials (RCTs) evaluating different interventions for trigeminal neuralgia are scarce and often deficient in quality due to a high risk of bias, variations in intervention modalities or errors in diagnosis [8]. Consequently, the lack of sufficient evidence makes clinical decision-making difficult.
Therefore, this systematic mapping review aims to provide a comprehensive overview of the research on trigeminal neuralgia, identify research gaps, and gather evidence for future research directions. The final product of this study will be a classified compilation of publications in the research domain, offering a visual guide for researchers, patients, and health professionals on the evidence available regarding treatment options and their reported outcomes. For this purpose, we have concentrated on systematic reviews (SRs) and randomized controlled trials, which offer the most reliable evidence on the effectiveness of treatment alternatives.
Objective
A mapping review will be conducted to systematically organize the evidence from different pharmacological interventions and surgical options for treating trigeminal neuralgia. Moreover, it will identify any existing gaps in knowledge of surgical procedures and pharmacological treatments. To address the mentioned objective, the following research question was formulated: “What are the pharmacological and surgical interventions for treating trigeminal neuralgia?”.
Methods
This systematic mapping review was drafted using the Preferred Reporting Items for Scoping Review (PRISMA-ScR) [12]. It adheres to the Global Evidence Mapping Initiative methods (GEM) [13], incorporating the quality of supporting evidence.
Eligibility criteria
The population, intervention, comparison, and outcomes (PICO) framework will be used to guide the eligibility criteria.
Study design: It will include systematic reviews including trials providing information on the pharmacological and surgical alternatives for the treatment of trigeminal neuralgia. Given the lack of consensus on the definition, a systematic review will be defined as a publication where the following are provided:
-
An explicit search in at least one electronic database.
-
A detailed description of the methods with explicit selection criteria.
-
A summary of the included studies either in narrative or quantitative format (such as a meta-analysis).
Rapid reviews will be eligible for inclusion if they meet our pre-defined inclusion criteria. Primary studies corresponding to RCTs will also be included. Only studies published as full texts in scientific journals will be considered for inclusion. Narrative reviews, broad synthesis studies, and observational primary studies will be excluded.
Population: Participants diagnosed with trigeminal neuralgia according to the International Headache Society (IHS) criteria [14].
Interventions: Treatment interventions, including medical and surgical alternatives, will be considered. The use of systemic and topical medicines and botulin toxin will be included in medical management and all the ablative techniques, neurosurgical procedures (MVD), and laser treatment in surgical management.
Comparison: It will include studies in which the control group receives a placebo, no treatment, or an active comparison different from the intervention group. Studies without a comparison group will be excluded.
Outcomes: The ‘TN Core Outcome Set’ (TRINCOS) domains will be considered to identify outcomes reported from RCTs and SRs. It considers the following domains: 1) Pain relief; 2) Duration of pain relief; 3) Pain intensity; 4) Pain interference; 5) Pain-free on medication; 6) Health-related quality of life; 7) Ability to participate in social roles and activities; 8) Overall response to treatment; 9)Satisfaction with treatment; 10) Side effects of medication and 11) Side effects of surgery [15].
Search and selection of the studies
Search and sources of information
A systematic search of the literature will be performed in different sources of information including the Epistemonikos database of Systematic Reviews, MEDLINE (via Pubmed), CENTRAL, and EBSCO to identify potentially eligible systematic reviews and trials on pharmacological and neurosurgical interventions for the treatment of trigeminal neuralgia. The search strategy will be adapted for each database, considering the differences in controlled vocabulary and syntax rules. No date or language restrictions will be applied. The search strategy developed is described in the Appendix. No date or language restrictions will be applied.
Selection of studies
Two independent reviewers will screen the retrieved references by title and abstract. Full-text selection of the included articles will be performed by duplicate. Studies not meeting the inclusion criteria will be recorded with reasons for their exclusion. Disagreements between reviewers of the included articles will be resolved by consensus or, if necessary, by a third author.
Data collection and extraction
Data extraction from the included articles will be performed using a standardized form. The following information will be extracted for each reference: general characteristics of the study (country, number of participants, mean age, female proportion), population (type of trigeminal neuralgia and diagnosis), interventions, comparisons, and outcomes measured.
Methodological quality evaluation
Two researchers will assess independently the quality of the included reviews and randomized controlled trials. The AMSTAR tool 2 will be used for systematic reviews, enabling a more detailed assessment of systematic reviews that include randomized trials [16,17]. The adherence will be rated to each item as follows: yes, partial yes, no or not applicable. The overall confidence in the results of the review will be rated as “critically low”, “low”, “moderate” or “high”, according to the AMSTAR 2 guidance based on seven critical domains. Reviews that scored “low” or “very low” will be considered as having major limitations.
For randomized trials, the risk of bias RoB-2 tool developed by the Cochrane Collaboration [18] will be used. The assessment includes bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in the measurement of the outcome and bias in the selection of the reported result. The judgments for each aspect will categorize the risk of bias as either high, low, or unclear. Any disagreements that arise during the evaluation process will be resolved through discussion between the two reviewers. If needed, a third author will be consulted to reach a resolution.
Data synthesis and analysis
The results will be summarized using descriptive statistics and tables. Absolute frequencies and proportions will be calculated for intervention variables.
Expected results
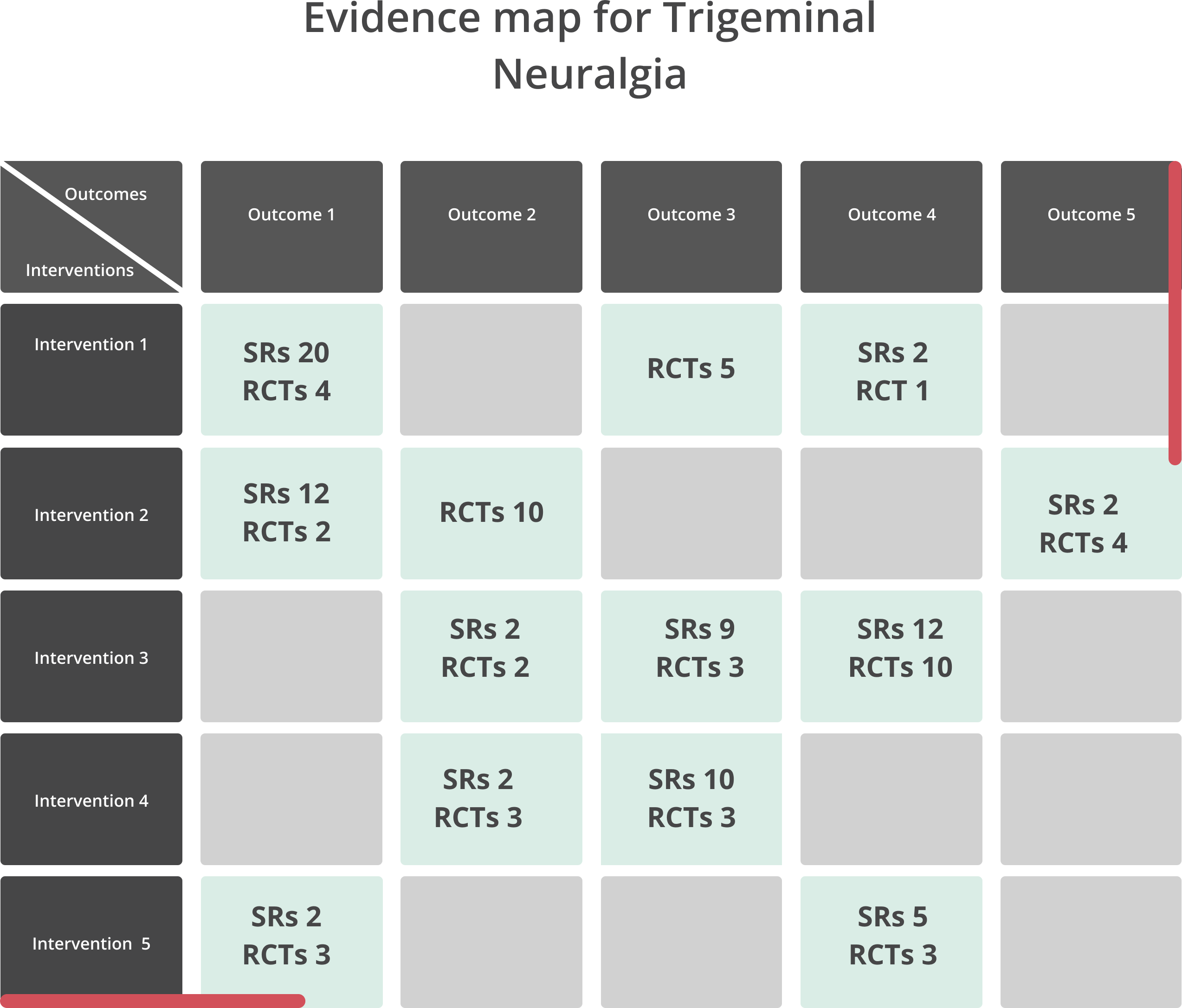
A PRISMA flowchart will be displayed to describe the process of selected studies. Descriptive tables will be developed to report the general characteristics of the systematic reviews and trials included. An evidence map will be created to depict the available evidence (Figure 1). All interventions reviewed in the included studies will be listed in rows and outcomes reported will be listed in columns. The number of reviews and trials will be reported for each cell of the map that connects one intervention to one outcome. Additionally, colors and figures will be used to describe the risk of bias in the included studies.
Illustrates the evidence map. Each box displays the number of systematic reviews and randomized controlled trials that matched each intervention with a specific outcome.
This study will hold significant importance within the research field of trigeminal neuralgia as it aims to identify and organize the existing evidence for treatment alternatives and their reported outcomes. By doing so, the review will help reduce research waste and provide a clear direction for future studies aimed at answering unresolved questions. The findings of this study are expected to streamline research efforts and optimize the use of resources, thereby accelerating progress in identifying effective treatments for trigeminal neuralgia.

