Protocolo
← vista completaPublicado el 16 de abril de 2021 | http://doi.org/10.5867/medwave.2021.03.8159
Efectos de la alianza terapéutica en los resultados clínicos en pacientes con osteoartritis de rodilla sintomática sometidos a un programa de ejercicio: protocolo de ensayo clínico aleatorizado
Effects of therapeutic alliance on clinical outcomes in patients with symptomatic knee osteoarthritis undergoing an exercise program: A randomized clinical trial protocol
Abstract
Background Previous evidence has shown that seniors physical therapists applying electrotherapy and an enhanced therapeutic alliance in their sessions can positively influence the levels of analgesia of patients with chronic low back pain. It is currently unknown if these effects can be achieved in people with symptomatic knee osteoarthritis when receiving treatment focused on therapeutic exercise.
Aim To determine the effects of different therapeutic alliance levels during the application of a therapeutic exercise program on pain intensity and pressure pain threshold in patients with symptomatic knee osteoarthritis.
Method This will be a randomized, parallel, two-arm, clinical trial. An intervention of three sessions of therapeutic exercise will be applied for one week. Patients aged 45 to 65 years old with a clinical and radiographic diagnosis of knee osteoarthritis will participate. Also, patients with a pain intensity of at least three months duration and 3 to 8 points in a numerical rating scale will be included. Patients will be randomly assigned to a therapeutic exercise experimental group with an enhanced therapeutic alliance (e.g., active listening, personalized conversation, empathy) or limited therapeutic alliance (e.g., one-way verbalization, brief interaction). Physical therapists will be trained in delivering these two levels of the therapeutic alliance. The pressure pain thresholds at the symptomatic knee and the pain intensity will be measured before and after the intervention.
Discussion The results of this research will determine the impact of the therapeutic alliance as a nonspecific relevant factor during the application of a therapeutic exercise program in the treatment of patients with symptomatic knee osteoarthritis.
Clinical trials registration number NCT04390932
Main messages
|
Introduction
Background and rationale
Osteoarthritis is one of the leading causes of pain [1] and disability globally [2]. The presentation and clinical progression of osteoarthritis are heterogeneous [3]. In this regard, the proportion of people with radiographic abnormalities and knee pain varies between 15% and 81%, reflecting a weak relationship between imaging findings and pain in people with symptomatic knee osteoarthritis [4],[5]. Pathological aging and comorbidities in the population have shown a strong correlation with knee pain and disability [6]. However, contrary to what was thought, the highest prevalence of symptomatic knee osteoarthritis is found in people younger than 65 years [5].
Contemporary clinical management of symptomatic knee osteoarthritis is multimodal [7]. For example, therapeutic exercise has been shown to effectively reduce pain, improve function, and reduce the intensity of symptomatic exacerbations and stiffness in people with knee osteoarthritis [8],[9]. Exercise modalities that have been proven to be effective in osteoarthritis include isometric [10], resistance [11], and aerobic [12] exercises. However, no modality is superior to the others, and combinations between them significantly improve outcomes [8],[13]. It has been observed that factors such as the therapist characteristics, the patient-therapist relationship, and the clinical environment may influence clinical interventions and may explain the variability of results in people with osteoarthritis [14]. Interestingly, it has been observed that supervised exercises are more effective than unsupervised ones, as well as exercise protocols that are accompanied by reinforcement sessions conducted by a physical therapist [15],[16], which could indicate a positive influence of the patient-therapist interaction in the results of rehabilitation.
The therapeutic alliance is the working relationship or positive social bond between the patient and the therapist based on collaboration, communication, empathy, and mutual respect [17]. The Enhanced therapeutic alliance is defined as the maximization of the therapeutic bond through communicational and behavioral strategies by the therapist. The therapeutic alliance has been positively correlated with decreased pain, disability, and adherence to exercise in people with low back pain [18],[19]. In addition, a recent systematic review demonstrated that an enhanced therapeutic alliance can improve rehabilitation outcomes, mainly in people with low back pain [20]. Nevertheless, these results are based on a small body of research with a high risk of bias.
Objectives
Therapeutic exercise is an inexpensive, accessible, and simple-to-apply intervention strategy associated with positive knee osteoarthritis outcomes [8],[9]. However, the influence of therapeutic alliance on clinical outcomes in this population receiving an exercise-based intervention is unknown. Therefore, our research will aim to determine the effects of different therapeutic alliance levels during the application of a therapeutic exercise program on pain intensity and pressure pain thresholds in people with symptomatic knee osteoarthritis who are treated in the Rehabilitation Unit of the Catholic University of Temuco. We hypothesized that the delivery of an exercise-based intervention along with an enhanced therapeutic alliance would lead to better clinical outcomes than the same program applied in a limited therapeutic alliance.
Trial Design
We will do a randomized, parallel, two-arm clinical trial, including three therapeutic exercise sessions within a one-week intervention period. Participants will be randomly assigned to the therapeutic exercise group with an enhanced therapeutic alliance or the therapeutic exercise group with a limited therapeutic alliance.
Methods
Study Setting
The study will be carried out at the Rehabilitation Unit of the Faculty of Health Sciences of the Catholic University of Temuco, Chile.
Eligibility criteria
This study will recruit a total of 48 participants (n = 24 per group). Candidates will be patients between 45 and 65 years old with a clinical and radiographic diagnosis of symptomatic knee osteoarthritis (grade 1-3 according to Kellgren and Lawrence [21]), with a pain intensity between 4 and 7 points on the Pain Intensity Numerical Rating Scale (PI-NRS) with at least three months duration [22].
Exclusion criteria are patients with other musculoskeletal, neurological, or immune conditions that cause pain or functional limitation in the lower extremities (hip osteoarthritis, patellar tendinopathy, sprains, radiculopathies, rheumatoid arthritis, or systemic lupus erythematosus); those with a history of trauma less than a year ago (fractures, meniscal or ligamentous knee tears); with surgical interventions of any kind on the lower limb; with knee mobility less than ninety degrees of flexion; in treatment with oral or intra-articular corticosteroids, antibiotics or exogenous opioids of any kind; or with unstable cardiovascular, respiratory, systemic or metabolic conditions that do not allow the performance of a physical exercise.
Interventions
Two physical therapists with two to three years of clinical experience in musculoskeletal rehabilitation will perform all interventions. They will be trained to conduct two different levels of therapeutic alliance during therapeutic exercise interventions. The training will include theoretical-practical training through lectures, demonstrative workshops, role-playing, and clinical simulation of the intervention protocols. This training is based on Fuentes et al. and Vong et al. [23],[24], consisting of eight teaching hours divided into two 4-hour sessions on different days to facilitate learning.
Based on the study by Fuentes et al. [23], and aiming to standardize physical therapists’ clinical care behavior, two therapeutic scripts will be created to guarantee the development of two different levels of therapeutic alliance during the interventions (Appendix 1). This clinical trial’s scripts differ from the one used by Fuentes et al. [20], as the present incorporates non-verbal language cues during the therapist-patient interaction, such as eye contact, postural adjustments, nodding, and other gestures. This standardization of non-verbal language was based on the study by Mollie et al. [25]. Speech-language therapists have provided consultancy to determine key aspects of communication that could influence the development of an enhanced therapeutic alliance and a limited therapeutic alliance. The manipulation of verbal and non-verbal aspects of communication was carried out according to the objective of each of the three intervention sessions: (1) Establish a bond and prescribe exercise; (2) Explain the mechanisms of action of exercise; (3) Reinforce the prescription and the mechanisms of action of the exercise. Also, the use of positive verbal suggestion during the explanation of the effects of the therapeutic exercise protocol was avoided in the scripts, because it has been observed to promote expectations that may influence treatment outcomes [26].
Each therapeutic script will have three stages, one for each intervention session, which will present a progressive and structured development to replicate pragmatic clinical patient care. The therapeutic script to promote an enhanced therapeutic alliance will be characterized by the personalization of the conversation, the verbalization of joint participation in the process, the presence of the professional in the development of the exercise protocol, and the frequent use of body language that transmits confidence, safety, attention, and active listening. The therapeutic script to promote a limited therapeutic alliance will not consider a personalization of the conversation but will use a unidirectional verbalization of the instructions. The professional will be absent intermittently during the therapeutic exercise sessions and will use neutral or negative non-verbal language cues.
Therapeutic exercise protocol
Both intervention groups will receive the same therapeutic exercise protocol, including aerobic, isometric, and isotonic exercises. These are effective in reducing pain and improving function in people with knee osteoarthritis (Appendix 2) [8],[12],[27]. The protocol will consist of three sessions of 45 to 60 minutes of supervised therapeutic exercise divided between intrasession stages. First, the aerobic exercise will be performed on a cycle ergometer for 20 minutes at an intensity of 50 to 60% of the maximum heart rate [28],[29], followed by joint mobility exercises with emphasis on ankle dorsiflexion and hip and knee flexion [30]. Finally, a repetition of isometric quadriceps exercises with knee extension in an open kinetic chain in a seated position with a weight of 10% of the maximum isometric force at the distal end of the leg, similar to what Fingleton et al. performed in people with knee osteoarthritis [31]. The second part of the exercise program will focus on strengthening the gluteus medius, gluteus maximus, and quadriceps muscles [32],[33],[34], for which three sets of four isotonic exercises will be performed, with repetitions until reaching a 7/10 Borg CR-10 [35].
Restrictions for the participants
Participants will be asked to avoid doing any intense physical activity or performing it at unusual levels, as well as stopping the use of analgesics for at least two days before the initial evaluation and throughout the development of this research. The use of rescue analgesics will be allowed only in flare-ups (increased pain over 8/10 Pain Intensity Numerical Rating Scale). Participants will also be asked to refrain from receiving other concurrent clinical interventions that may influence the results, such as physical therapy, massage therapy, electrotherapy, osteopathy, chiropractic, among others. To control the patients’ physical activity, use of rescue analgesics, and interventions with other professionals, a monitoring form has been developed that they must complete before each session.
Primary outcome measures
Pain intensity and pressure pain thresholds will be evaluated 48 hours before starting the therapeutic exercise protocol and 48 hours after finishing the three intervention sessions. According to the patient’s report, the evaluation of the limb with the more severe symptoms will be assessed in the case of bilateral knee pain.
The researcher will evaluate volunteers immediately after signing the informed consent and 48 hours after completing the intervention protocol. This evaluation consists of recording each participant’s characteristics and determining the baseline of the different variables to be measured (pain intensity, pressure pain threshold, physical function, expectations, and conditioned pain modulation). The researcher received a training in pressure algometry, demonstrating excellent intra-examiner reliability in a previous pilot study (intraclass correlation coefficient = 0.86; 95% confidence interval: 0.65 to 0.96).
Pain intensity. Continuous quantitative variable to be evaluated through the numerical pain rating scale. The Pain Intensity Numerical Rating Scale is a valid and reliable tool for self-reporting pain intensity of 11 points (0 - 10) [36]. The clinically important minimal difference for Pain Intensity Numerical Rating Scale in people with knee osteoarthritis is 1.08 to 3.66 [37],[38].
Pressure Pain Threshold (PPT). Continuous quantitative variable to be evaluated through a Wagner FPX25 pressure algometer in the articular interline of the symptomatic knee. Pressure algometry has proven to be a reliable tool for measuring pressure pain thresholds in people with knee osteoarthritis [39],[40]. The minimum significant clinical difference has been reported to be > 1.1 kilograms/square centimeter/second [41].
Secondary outcomes
Lower limb function. Discrete quantitative variable to be evaluated with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC). This index is a valid and reliable instrument to assess pain, stiffness, and physical function in people with knee osteoarthritis [42]. This outcome will be assessed at baseline.
Therapeutic Alliance. Discrete quantitative variable to be evaluated through the Work Alliance Subscale of the Pain Rehabilitation Expectations Scale (PRES). This is a valid and reliable clinical tool, and it is the only scale to date that evaluates the 8 dimensions of the therapeutic alliance [17],[43]. This outcome will be assessed post-intervention.
Expectations. Discrete quantitative variable to be evaluated through the Credibility and Expectations Questionnaire (CEQ). It is a valid and reliable instrument composed of 6 items, proven clinically useful in people with knee osteoarthritis [44],[45]. This outcome will be assessed at baseline.
Perception of improvement or deterioration. Discrete quantitative variable to be evaluated through the Global rating of change scale (GROC), in its Spanish version [46]. In rehabilitation, the assessment of the perception of change by the patient is relevant. Changes in the Global rating of change scale will be interpreted as an indicator of clinical significance by the patient. This will be measured after each treatment session.
Conditioned pain modulation. Continuous quantitative variable that based on the performance of pressure algometry before and after applicating a conditioning stimulus (noxious) in the upper limb [47]. This paradigm assesses the indemnity of endogenous analgesia mechanisms, which have been related to the hypoalgesic response with exercise [48]. For this research, as a conditioning stimulus, the contralateral hand to the symptomatic knee will be immersed in cold for 1 minute, with the pre and post evaluation of the pressure pain threshold in the ipsilateral forearm and the articular interline of the symptomatic knee [47],[49]. Through a cold thermal stimulus, the conditioned assessment of pain presents excellent intrasession reliability, with an intraclass correlation coefficient of 0.85[49]. This outcome will be assessed at baseline.
Anxiety and depression. Discrete quantitative variable, which will be evaluated through the Hospital Anxiety and Depression Scale (HADS), a reliable instrument that evaluates the emotional state of outpatients [50]. The scale comprises 14 items organized into two subscales that allow an independent assessment of anxiety and depression. The Hospital Anxiety and Depression Scale is currently validated in Chile [51]. This outcome will be assessed at baseline.
Adherence of the physical therapist to the therapeutic script. Adherence of the clinicians to protocols was based on how closely they followed the established procedure. This will be based on videotaping all treatment sessions, of which 20% will be randomly selected for evaluation. Two research assistants not involved with the trial will separately rate each session regarding treatment fidelity using an evaluation form designed explicitly for this study.
Participant timeline
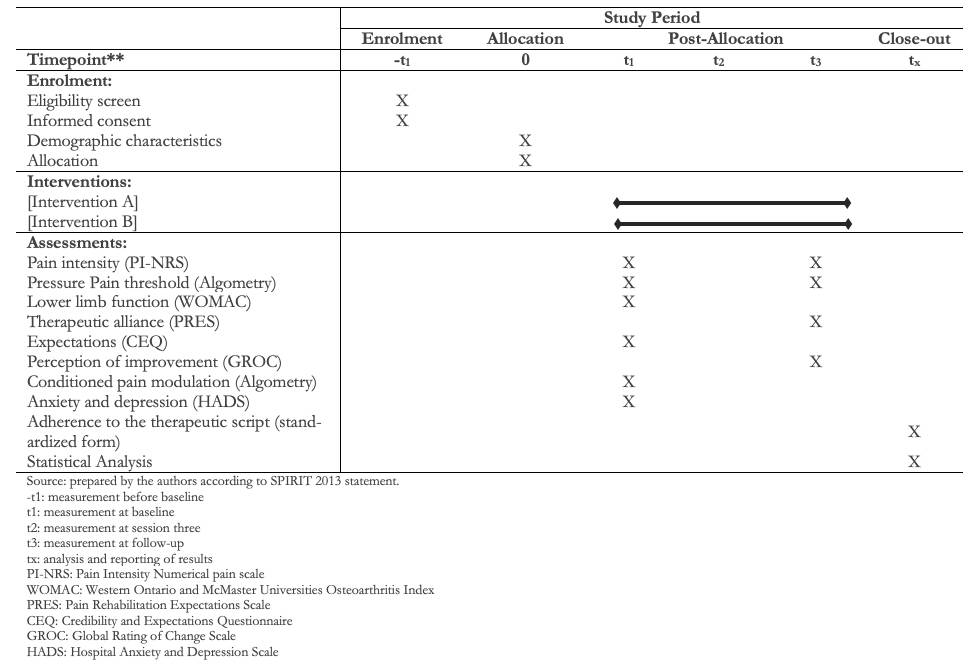 Full size
Full size Sample size
The sample size calculation was conducted through the G*Power 3.1.9.2 software and considered the use of the MANOVA test of special effects and interactions, with two primary variables and seven secondary variables, using a moderate effect size (d = 0.48) for the Pressure Pain Thresholdaccording to Fuentes et al.[23], an alpha of 0.05 and a statistical power of 80%. Finally, considering a likely 10% loss to follow-up, the sample size required for this research was 48 people in total.
Recruitment
Participants will be recruited in primary health care centers, social networks, and radio and television broadcasting. Potential participants will be called to an eligibility assessment session, where they will be explained the nature and development of the research, the informed consent, and the eligibility criteria. Patients who voluntarily agree to participate in this research and meet the eligibility criteria will have to confirm this by signing the informed consent.
Sequence generation and concealment
Participants will be assigned to an enhanced therapeutic alliance or a limited therapeutic alliance group. Simple randomization using an online program will be performed by an external research assistant. In addition, the allocation will be concealed by the method of the opaque sealed and consecutively numbered envelopes by the same external assistant. Figure 1 shows the study timeline.
Blinding (masking)
All pre and post-intervention outcome measures will be evaluated by a physical therapist blinded to the group assignment of participants and who will not participate in applying the intervention protocol. Participants will be blinded to the level therapeutic alliance and hypothesis and will only be informed that they have an equal chance of being assigned to either group. Data analysis will be performed by a statistical advisor external to the study and blinded to group assignment.
Data collection
A pre-designed form will be used to establish the baseline and evaluate the post-intervention, which considers demographic data such as age, gender, and education. Regarding clinical variables, data to be included in the form are: comorbidities, duration of symptoms in the knee, blood pressure, body mass index (weight, height), unilateral or bilateral diagnosis of knee osteoarthritis, the severity of degenerative joint, sleep disorder, and isometric quadriceps muscle strength. The selection of the demographic and clinical history of the evaluation form was based on the recommendations of the IMMPACT group for the correct phenotyping of participants in pain-related clinical trials[52].
The two clinical physical therapists will track a history of the administration of the exercise protocol in a data collection sheet in each session. This sheet considers data such as the patient’s general condition, the dosage of each exercise, and the control of variables such as pain intensity (PI-NRS) and the perception of improvement or deterioration (GROC).
Data management
All information related to the study will be stored safely in the Rehabilitation Unit of the Catholic University of Temuco in closed filing cabinets with limited access. The information of each participant will be classified through alphanumeric codes. The files will be kept in storage for two years after the end of the study, and forms, hard drives and pen drives will be kept in locked cabinets. Access to the data will be restricted to personnel outside the investigation. Additionally, a backup of the data will be made.
Statistical analysis
Participant characteristics in the two treatment groups will be compared using independent t-tests and chi-square tests for continuous and categorical variables.
The normality of the data will be evaluated through the Shapiro Wilk test, while the evaluation of the differences in the intragroup and intergroup primary outcomes of PI-NRS and PPT, and the interaction of these with the secondary outcome measures, will be performed through the MANOVA test of special effects and interactions with Games-Howell post hoc. The level of significance was established at α = 0.05. Subsequently, the Cohen d will be calculated to determine the intervention’s effect size on each primary outcome. The results of adherence to therapeutic alliance protocols will be reported with descriptive statistics. The degree of agreement in evaluating adherence to therapeutic alliance protocols between the external assistants will be carried out through the Intraclass correlation coefficient (ICC) statistic. All the data analysis of the data will follow the intention to treat principle. Records of the last observation carried forward (LOCF) will be used to impute missing data. The IBM SPSS Statistics software, version 24.0 will be used for all analyses.
Harms
The therapeutic exercise protocol has a low probability of increasing pain. However, if this situation occurs, the therapist will make an immediate evaluation to determine the cause and provide the necessary assistance (immediate management of pain with physical agents or referral to the physician). All adverse events will be reported on the participant’s monitoring form.
Ethics and dissemination
This randomized clinical trial protocol has been approved by the scientific ethics committee of the Catholic University of Maule, Talca, Chile (266/2019). All patients who meet the eligibility criteria will be invited to participate in this research. All participants will know in detail the research procedures and will give their consent in a standard form. This research protocol has been registered before the recruitment of the participants at www.clinicaltrials.gov (NCT04390932).
The data collected in this investigation will be available only to members of the investigation team. Dissemination of the research results will not include any identifying data of study participants. This project’s results will be disseminated to educational institutions and health professionals who could potentially benefit from these results. The study results will also be presented at national and international conferences and published in scientific journals. Participants may request information on their results at any time during the investigation. Participants will receive by email a report of their results and the articles published resulting from this research.
Discussion
Clinical evidence shows that pain depends on the context, and this can be modified by contextual factors such as the therapeutic alliance between the physical therapist and the patient. Therefore, promoting an enhanced therapeutic alliance is in line with contemporary patient-centered approaches, which seek an individualized treatment that considers the patient´s expectations, a consensus in decision-making, and strengthening the therapist-patient relationship[53].
Some mechanisms have been proposed to explain the positive effects of the therapeutic alliance on treatment outcomes. Evidence has shed light on the neurobiology of the clinician-patient relationship and the mechanism of how appropriate words from the clinician can induce meaningful changes in neural activity, leading to the activation of the endogenous opioid system, biological changes, and improved pain outcomes[54],[55],[56],[57],[58]. For example, it has been shown that when the clinician-patient relationship is compromised, the analgesic response magnitude is reduced[59],[60]. Reduction of the therapeutic alliance negatively influences pain outcomes, possibly due to reduced activation of opioid mechanism in patients in the absence of the doctor, nurse or physiotherapist during the clinical procedure[23],[55]. Other suggest that three possible ways the relationship augments the response to a positive therapeutic context include counteracting loneliness, increasing expectations of relief, co-regulating emotions, and decreasing arousal [61].
As the therapeutic alliance seems to influence treatment responsiveness, physical therapists should maximize it during their interventions when dealing with painful conditions. Physical therapist should develop social and communication skills, including empathy, active listening, emotional support, and verbal and non-verbal language, to positively influence through contextual factors the therapeutic alliance. Accordingly, patients may be actively incorporated in their rehabilitation process, leading to better clinical outcomes [17],[53].
In other disciplines, therapeutic alliance has been positively associated with greater adherence and satisfaction and better clinical outcomes in patients [62],[63],[64]. Similar results have been observed in physical therapy in studies of limited methodological quality, where the therapeutic alliance has been considered a dependent variable [18],[65]. Also, therapeutic alliance has been shown to predict the outcome of the evolution of function, pain, and disability and improve satisfaction and adherence in people with musculoskeletal disorders who receive physical therapy [18],[65]. The findings of these investigations are encouraging; however, additional research in the area is warranted.
The therapeutic alliance between patient and clinician appears to be a powerful influence in conditions such as chronic low back pain, and physiotherapists need to maximize that power to reduce individuals’ suffering. Efforts to enhance patient-clinician communication and systematically examine nonspecific treatment factors are likely to promote effective chronic pain management. These results have important implications, namely that factors other than the specific treatment may have a prominent role in achieving positive clinical outcomes—exploring them is central to advancing physiotherapy practice. However, further research is warranted, mainly to confirm the beneficial effects of enhanced therapeutic alliance in other musculoskeletal conditions or patients receiving different interventions (e.g., therapeutic exercise). Data from a broader spectrum of musculoskeletal disorders, such as knee osteoarthritis and acute or chronic conditions, would further elucidate the significance of the therapeutic alliance in managing musculoskeletal pain. Future research in this area may provide a helpful framework to understand and increment the placebo effects and the therapeutic relationship (therapeutic alliance) in physiotherapy.
Strength and limitations
To our knowledge, this is the first randomized controlled study aimed at exploring the effects of manipulating the therapeutic alliance in physiotherapy treatment in knee osteoarthritis. The testing protocol will be standardized to minimize bias and conducted in the rehabilitation unit of our university. The latter may offer an environment somewhat different from routine clinical practice. There are many characteristics of the design that may strengthen our results. Our trial is designed to have a high internal validity as shown by adequate randomization, concealed allocation, baseline comparability among groups, and blinding of the research team. Clinicians will deliver the interventions following a highly standardized study protocol designed to deliver different therapeutic contexts. Also, this design protocol aims to test the immediate effect of enhanced therapeutic alliance and its effect over a series of treatment sessions.
However, any inference from this study needs to be tempered due to some limitations. First, since the design did not include a no-treatment control group, the study results might be under scrutiny. To avoid the confounding effect of statistical phenomenon such as regression to the mean or the spontaneous temporal variation of pain on the therapeutic effect of the therapeutic alliance, clinical trials need to include an untreated group (that is, natural history) [66]. Second, our study protocol includes a short-term follow-up. It would be interesting to clarify if the effects of the therapeutic alliance could be sustained in a longer term. Third, this research will measure the effect of therapeutic alliance on subjective outcomes (that is, PI-NRS, PPT). Therefore, future research should be focused on the analysis of therapeutic alliance mechanisms in objective outcomes such as the modification of pain-relieving peptides or changes in cerebral activity (that is, neuroimaging) in areas linked with pain processing and pain modulation in patients suffering from chronic musculoskeletal pain.
Thus, future research is needed to overcome these limitations and expand the existing evidence regarding the effects of the therapeutic alliance as another therapeutic agent within clinical practice.
Notes
Authorship contributions
IC-V: conceptualization, methodology, investigation, project administration, writing- original draft preparation, writing- reviewing and editing. FJ: conceptualization, methodology, supervision, writing - review and editing.
Competing interests
The authors declare that they have no potential conflict of interest concerning the research, authorship, or publication of this article.
Funding
This project was funded by “Vicerrectoría de Investigación y Postgrado de la Universidad Católica de Temuco (VIPUCT N° 2020EM-IC-01)”. Mr. Cuyul-Vásquez is supported by the Government of Chile through the National Agency of Research and Development (ANID) Master Scholarship.
Clinical trials registration number
NCT04390932
Ethics
The protocol was approved by the scientific ethics committee of the Catholic University of Maule, Talca (266/2019).
From the editors
The peer-review process was done on the submission of this English version. The Journal has copyedited the article.
Appendix
Appendix 1.
Appendix 2.

