Revisión sistemática
← vista completaPublicado el 14 de marzo de 2018 | http://doi.org/10.5867/medwave.2018.02.7179
Eficacia del tratamiento con omeprazol y bicarbonato de sodio en la enfermedad por reflujo gastroesofágico: revisión sistemática
Efficacy of omeprazole/sodium bicarbonate treatment in gastroesophageal reflux disease: a systematic review
Abstract
BACKGROUND Proton pump inhibitors are the most effective medical therapy for gastroesophageal reflux disease, but their onset of action may be slow.
OBJECTIVES To assess the available literature regarding the efficacy of omeprazole/sodium bicarbonate in gastroesophageal reflux patients.
METHODS A systematic review was conducted. A systematic literature search starting from 2000. Reviewed manuscripts concerning the effectiveness of omeprazole/sodium bicarbonate treatment in gastroesophageal reflux disease were reviewed and the data were extracted. Data were subsequently analyzed with descriptive statistics.
RESULTS This review included information of four studies. Two trials compared the efficacy of omeprazole/sodium bicarbonate versus omeprazole. One study compared the efficacy of once-daily morning or nighttime dosing. And another study compared omeprazole/sodium bicarbonate/alginate versus omeprazole. In total, there was no difference between omeprazole/sodium bicarbonate and omeprazole. However, there is a trend towards more sustained response and a greater proportion of patients with sustained total relief by 30 minutes with omeprazole/sodium bicarbonate.
CONCLUSION Omeprazole/sodium bicarbonate therapy is not more effective than omeprazole in the treatment of gastroesophageal reflux disease. However, data obtained suggest that it can have a more sustained response and sustained total relief.
Introduction
According to the World Gastroenterology Organization Global Guidelines, gastroesophageal reflux disease is defined as troublesome symptoms sufficient to impair an individual’s quality of life, or injury or complications that result from the retrograde flow of gastric contents into the esophagus, oropharynx, and/or respiratory tract [1]. Gastroesophageal reflux disease is widely prevalent around the world, with clear evidence of increasing prevalence in many developing countries [1]. In Mexico, the prevalence rates vary from 19.6% to 40.0%, depending on the methodology employed [2].
The three presentations of gastroesophageal reflux disease are erosive esophagitis, nonerosive reflux disease and Barrett's Esophagus [3].
Management of gastroesophageal reflux disease usually consists of life style interventions, the reduction of esophageal luminal acid by its local neutralization or through the suppression of the secretion of gastric acid by means of medical treatment and anti-reflux surgery [1].
Current guidelines consider proton pump inhibitors to be the most effective medical therapy for gastroesophageal reflux disease, owing to their profound and consistent suppression of gastric acid [1],[2],[4],[5],[6].
Only one proton pump inhibitor, omeprazole, has been combined with an antacid –sodium bicarbonate– in order to obtain faster absorption compared to the absorption obtained using delayed-release tablets. The fastest absorption has been associated with a faster start to the suppression of gastric acidity compared with that obtained using delayed-release tablets [7].
The association of omeprazole with sodium bicarbonate and sodium alginate is capable of an intense and significant suppression of acid in children with gastroesophageal reflux disease [8].
Objective
The aim of this systematic review was to critically assess the available literature on the effectiveness of the omeprazole/sodium bicarbonate combination versus omeprazole in adults with gastroesophageal reflux disease.
Design
This systematic review was conducted in accordance with the PRISMA (Preferred Reporting for Systematic Reviews and Meta-Analyses) guidelines [9]. The methodological approach included the development of selection criteria, definition of search strategies, assessment of the quality of the studies and the abstraction of the relevant information. Followed by the checklist of PRISMA statements [9].
Criteria for the inclusion of studies
The criteria for the inclusion of studies were defined before starting to collect the information for the suitable identification of the studies that were eligible for analysis. All the studies whose primary objective was to assess the effectiveness of omeprazole/sodium bicarbonate as a treatment for gastroesophageal reflux disease in adults were obtained and analyzed to verify if they met the following selection criteria.
Types of study: Every type of prospective and retrospective clinical trial was eligible for inclusion without any limitation on the length of the trial. Studies consisting of the review, comments and summaries of conferences were not considered.
Types of participants: Adults with gastroesophageal reflux disease or Barrett's Esophagus.
Types of interventions: Use of omeprazole/sodium bicarbonate as a treatment for gastroesophageal reflux disease or Barrett's Esophagus.
Types of result measurements: The primary result was the effectiveness of the therapy determined by the success in the relief of the symptoms, improvement in pH readings and/or in the endoscopic evaluations in each study.
Data sources
Search strategy
A search was carried out for articles on gastroesophageal reflux disease and the omeprazole/sodium bicarbonate combination in PubMed/Medline, SciELO and LILACS, without any language restrictions. The search strategy was: "Omeprazole"[Mesh] OR "omeprazole, sodium bicarbonate drug combination"[All Fields] AND "Barrett’s Esophagus"[Mesh] OR "gastroesophageal reflux"[MeSH Terms] AND (Clinical Trial[ptyp] AND "loattrfull text"[sb] AND ("2000/01/01"[PDAT]: "2017/03/31"[PDAT]) AND "humans"[MeSH Terms] AND "adult"[MeSH Terms]) AND (Clinical Trial[ptyp] AND "loattrfull text"[sb] AND ("2000/01/01"[PDAT]: "2017/02/28"[PDAT]) AND "humans"[MeSH Terms] AND "adult"[MeSH Terms]) NOT ("Child"[Mesh]) NOT "Clinical Trials, Phase I as Topic"[Mesh].
Review methods
Assessment of the quality of the studies
All the studies were filtered according to their title and summary. The manual search included references from the articles that were obtained. The relevant full-length articles were obtained and assessed. Furthermore, the references included from the original articles were confirmed in order to complete the search. The articles were rated according to the levels of evidence for therapeutic studies of the Centre for Evidence-Based Medicine [10].
Data extraction
If the articles were eligible, the following information was obtained from the original articles: year, design of the study, institution, number of patients, pathology, intervention, comparator, assessment tool and main result. The effectiveness of the therapy was determined by the success in the relief of symptoms, improvement in pH readings or the endoscopic evaluations. The information was analyzed and reported in tables and text.
Results
The summarized search strategy identified 318 potentially relevant publications. After the selection of titles and studies, 302 were directly excluded because they were not specifically connected with the use of omeprazole or bicarbonate. This meant that 16 studies were selected for their review; 12 were not connected with a specific treatment, although they were connected with the pathology: fundoplication (4), metal anti-reflux valve (1), anti-reflux surgery (2), esomeprazole (3), esophageal stents (1) and an examination (1). Finally, four articles were selected for the final qualitative and quantitative review (Figure 1).
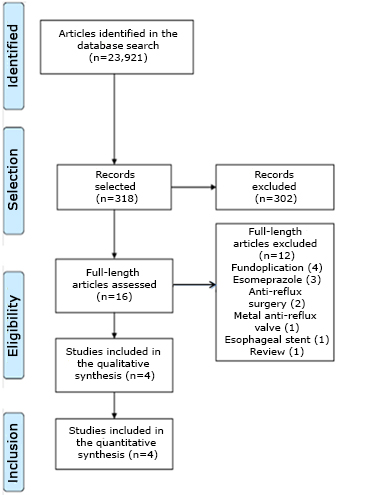 Full size
Full size Quality and methodology of the studies
There was significant heterogeneity in the methodology of the studies included in the final report. Three articles had a 1B level of evidence and the other, 2B. Three studies were clinical, controlled, random and prospective [11],[12],[13]. Two of them [12],[13] were open –one pilot study with a parallel group– [12] and the other was a cross-over study– [13]. Two studies were multi-centered [11],[13]. One study was for a cohort [14]. Three studies [11],[12],[13] were carried out in populations with gastroesophageal reflux disease and one in Barrett's Esophagus [14]. In three studies [11],[12],[13], the assessment of the symptoms was based on a questionnaire (different for each study).
Other assessment tools employed were esophagogastroduodenoscopy [11] and Bravo pH monitoring [14]. The gastroesophageal reflux disease diagnostic criteria for the three articles which were included were basically based on the typical symptoms associated with reflux (acidity and/or regurgitation) happening at least twice a week, although the length of time was not clearly specified in the three studies [11],[12],[13]. Three studies used omeprazole/sodium bicarbonate as their intervention [11],[12],[14] and one, omeprazole/sodium bicarbonate/alginate [13].
As for the dosage of omeprazole/sodium bicarbonate, one study used this agent at a dose of 20 mg once a day for three days [11], and another used 40 mg once a day for eight weeks [12]. In another study, 40 mg omeprazole/sodium bicarbonate twice a day for 28 days was prescribed [14]. One of the clinical trials used 20 mg daily for seven days followed by on demand [13]. Omeprazole was the comparator in two studies (Tables 1 and 2) [11],[13].
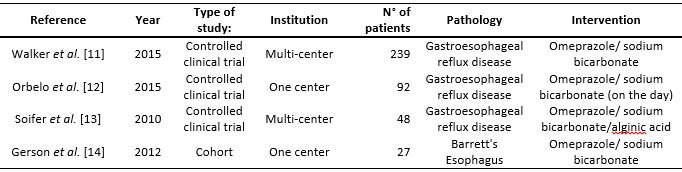 Full size
Full size 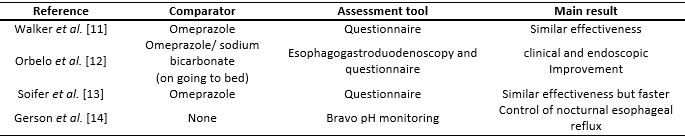 Full size
Full size Omeprazole versus omeprazole/sodium bicarbonate
Only one study compared the effectiveness of the omeprazole/sodium bicarbonate combination with monotherapy with omeprazole. It was designed as a randomized, multi-center, double-blind trial with double-dummy [11]. In total, 39 sites in six countries took part and they enrolled 262 patients and 239 were randomly chosen to receive omeprazole/sodium bicarbonate (n=122) or omeprazole (n=117). The basal and demographic characteristics were similar in both treatment groups. Both therapies were effective in reducing the severity of the acidity associated with gastroesophageal reflux disease. A sustained response was achieved in 75.2% of the group with omeprazole versus 81.1% of the omeprazole/sodium bicarbonate group in the modified intent-to-treat analysis (p=NS). There was a tendency towards more patients with a sustained response for 30 minutes and a higher ratio of patients with total relief sustained for 30 minutes in the omeprazole/sodium bicarbonate group. Both therapies had similar safety profiles.
Omeprazole/sodium bicarbonate
Regarding the omeprazole/sodium bicarbonate combination therapy, there are two relevant articles, one of which was an open, randomized pilot study of parallel groups, in one center [12], and one cohort study [14]. Orbelo et al. [12] carried out research in the Mayo Clinic about the effectiveness of the combination administered for eight weeks in the morning or at night to 92 patients with severe erosive esophagitis caused by reflux. This was followed up with an esophagogastroduodenoscopy and validated with self-report questionnaires. In total, 88% of the participants were healed or their erosions improved. There was no significant difference between the morning and nighttime administration in the variables studied (healing of the mucus, resolution of the symptoms or acid regurgitation).
In Gerson’s study, they enrolled 27 patients with Barrett's Esophagus, confirmed by endoscopy [14]. Of which, 15 completed the protocol. All the participants had their pH repeatedly measured with the administration of omeprazole/sodium bicarbonate before breakfast and before going to bed. Moreover, the impact of the symptoms of gastroesophageal reflux disease on health-related quality of life was measured, using the quality of life in reflux and dyspepsia questionnaire. All the patients achieved the normalization of their pH in a supine position and control of nocturnal esophageal reflux. There was an improvement in health-related quality of life.
Omeprazole/sodium bicarbonate/alginate
Soifer et al. designed an open, randomized, multi-center cross-over trial with 48 patients with nonerosive reflux disease [13]. The aim was to compare the effectiveness of an on-demand treatment with omeprazole/ sodium bicarbonate/alginic acid versus only omeprazole. Effectiveness was assessed by a questionnaire. Both therapies were equally effective, however, the combination therapy was faster at relieving the symptoms.
Because of the heterogeneity of the studies, it was impossible to get a summary of the patients treated.
Discussion and conclusions
This systematic review focused on assessing current knowledge about the omeprazole/sodium bicarbonate combination as a treatment for gastroesophageal reflux disease. This topic is divided into three parts:
- Omeprazole versus omeprazole/sodium bicarbonate.
- Omeprazole/sodium bicarbonate.
- Omeprazole/sodium bicarbonate/alginate.
The review consisted of a thorough examination of the literature and the description of every study that was found. Only four studies met the inclusion criteria. The differences in the methodology of the studies analyzed (different dosage plans, comparators and assessment tool) prevented from making a summary of the patients treated.
With these limitations, it is not possible to carry out an analysis of the studies assessed in this review. However, some considerations may be proposed. The little information available about the rate of response seems to be at least comparable to that for the standard treatment, omeprazole [11]. No statistical differences were found between the morning and nighttime dosage schedules [12].
Even more interesting are the results in terms of a trend towards a sustained response of total relief for 30 minutes in the omeprazole/sodium bicarbonate group, probably relating to the time to reach peak concentration, tmáx = 0,49 h, as demonstrated in the bioavailability studies [11],[15]. A similar effect was demonstrated in Soifer's study where omeprazole/sodium bicarbonate/alginate acted faster in the relief of the symptoms compared to omeprazole [13].
These results are consistent with the formulation –without enteric coating. This formulation employs sodium bicarbonate to protect the uncoated omeprazole from acid degradation. The potential advantage of this formulation us a faster absorption of omeprazole compared to delayed-release capsules. The fastest absorption has been associated with a faster start to the suppression of gastric acidity compared with that obtained using delayed-release capsules [16].
Conclusion
There is not much information about the effectiveness of the omeprazole/sodium bicarbonate combination on gastroesophageal reflux disease. Clearly, the heterogeneity of the studies makes the direct comparison with omeprazole difficult.
The results indicate that omeprazole/sodium bicarbonate could be useful for patients with gastroesophageal reflux disease, although it does achieve satisfactory effects. In fact, the type II error in studies should be considered as a reason for the failure to demonstrate a significant difference in the rate of responders. The number of patients may not have been sufficiently big to demonstrate it.
The evidence shown in this systematic review suggests that the omeprazole/sodium bicarbonate combination can have a faster start of action than omeprazole and this can benefit a particular population.
Additional clinical trials with a better design and a higher number of participants are needed to check the effectiveness of this combination therapy.
Notes
From the editor
The authors originally submitted this article in Spanish and subsequently translated it into English. The Journal has not copyedited this version.
Declaration of conflicts of interest
The author has completed the ICMJE's conflict of interest declaration form translated into Spanish by Medwave, and declares that she has not received funding for the report; has no financial relationships with organizations that might have an interest in the published article in the last three years; and has no other relationships or activities that could influence the published article. Forms can be requested by contacting the author responsible or the editorial management of the Journal.
Financing
The author states that the study was funded by Laboratorios Liomont S. A.

